Question: Modelingmhlem: Consider a CSTR reactor with ash product separation as shown in the schematic below. Fresh feed enters the reactor with a volumetric ow rate
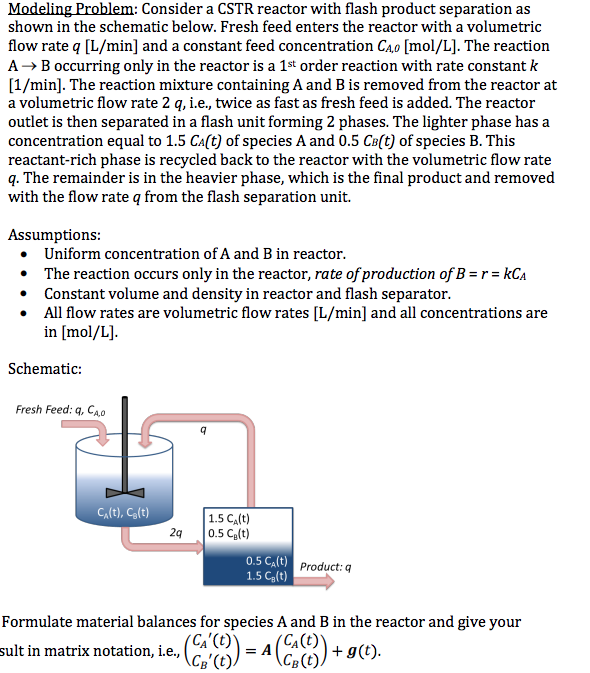
Modelingmhlem: Consider a CSTR reactor with ash product separation as shown in the schematic below. Fresh feed enters the reactor with a volumetric ow rate :3 [Lgr min] and a constant feed concentration the [molg' L]. The reaction A } E occurring only in the reactor is a lt order reaction with rate constant k [1 J{'min]. The reaction mixture containing A. and B is removed from the reactor at a volumetric ow rate 2 o, i.e., twice as fast as fresh feed is added. The reactor outlet is then separated in a ash unit forming 2 phases. The lighter phase has a concentration equal to 1.5 Eaftj of species a and 0.5 Heft} of species B. This reactant-rich phase is reqrcled hack to the reactor with the volumetric ow rate q. The remainder is in the heavier phase, which is the nal product and removed with the ow rate or from the ash separation unit. Assumptions: o Uniform concentration of a and E in reactor. I The reaction occurs only in the reactor, rote ofproducon ofH = r = kh i Constant volume and density in reactor and ash separator. a All ow rates are volumetric ow rates [Lfmin] and all concentrations are in [mo];r L]. Schematic: Fresh Feed: 1;, Eu Formulate material balances for species A and B in the reactor and give your EAT\") = A (EA [It] sult in matrix notation, i.e., (Eel (t) 1.730)) + .96)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


