Question: Module 8: 20 Century Physics Topic 1 Application: Wave-Particle Duality 1. Imagine that you are Robert Milliken, a physicist living in the early 1900s when
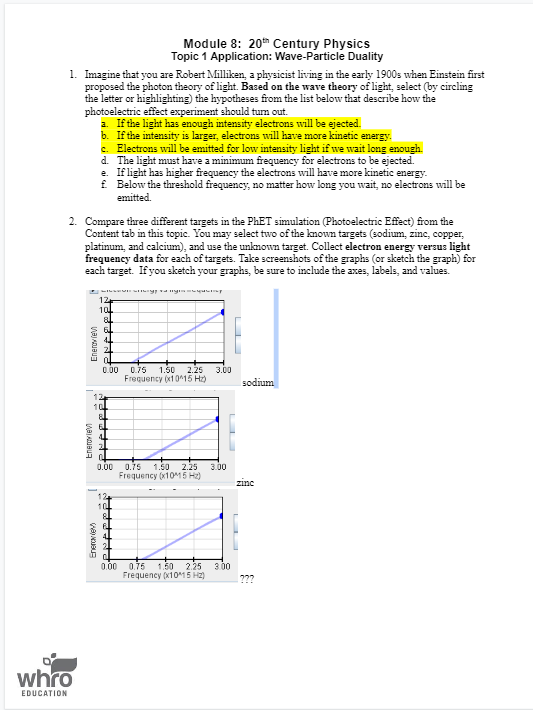
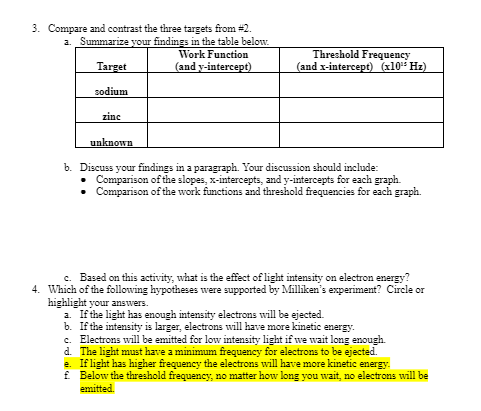
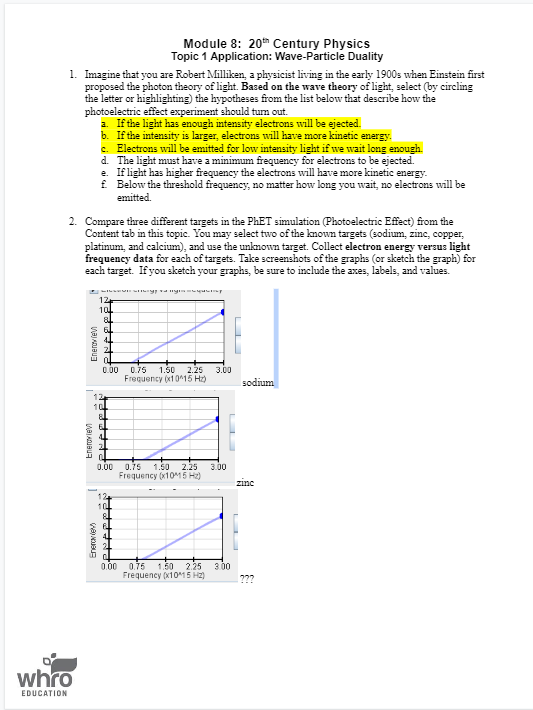
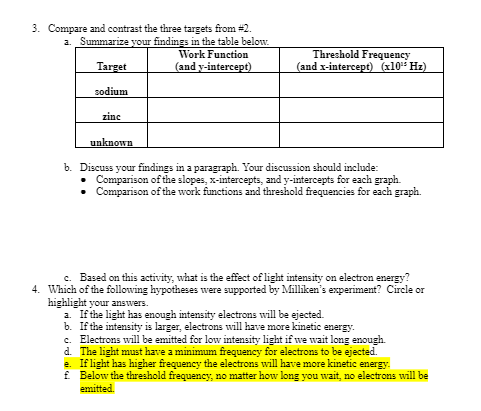
Module 8: 20" Century Physics Topic 1 Application: Wave-Particle Duality 1. Imagine that you are Robert Milliken, a physicist living in the early 1900s when Einstein first proposed the photon theory of light. Based on the wave theory of light, select (by circling the letter or highlighting) the hypotheses from the list below that describe how the photoelectric effect experiment should turn out. a. If the light has enough intensity electrons will be ejected. b. If the intensity is larger, electrons will have more kinetic energy. C. Electrons will be emitted for low intensity light if we wait long enough. d. The light must have a minimum frequency for electrons to be ejected. e. If light has higher frequency the electrons will have more kinetic energy. Below the threshold frequency, no matter how long you wait, no electrons will be emitted. 2. Compare three different targets in the PhET simulation (Photoelectric Effect) from the Content tab in this topic. You may select two of the known targets (sodium, zinc, copper, platinum, and calcium), and use the unknown target. Collect electron energy versus light frequency data for each of targets. Take screenshots of the graphs (or sketch the graph) for each target. If you sketch your graphs, be sure to include the axes, labels, and values. 12 Enerow levi 1 0.75 1.50 2.25 3.00 Frequency (x10*15 Hz) sodimm Enerovlevi 0.00 0.75 1.50 2.25 3.00 Frequency (x10 15 Hz) zinc Enercries 0.00 0.75 1.50 2.25 3.00 Frequency (x10*15 HZ) whro EDUCATION3. Compare and contrast the three targets from #2. a. Summarize your findings in the table below. Work Function Threshold Frequency Target (and y-intercept) (and x-intercept) (x10" Hz) sodium zinc unknown b. Discuss your findings in a paragraph. Your discussion should include: Comparison of the slopes, x-intercepts, and y-intercepts for each graph. . Comparison of the work functions and threshold frequencies for each graph. c. Based on this activity, what is the effect of light intensity on electron energy? 4. Which of the following hypotheses were supported by Milliken's experiment? Circle or highlight your answers. a. If the light has enough intensity electrons will be ejected. b. If the intensity is larger, electrons will have more kinetic energy. . Electrons will be emitted for low intensity light if we wait long enough. d. The light must have a minimum frequency for electrons to be ejected. 2. If light has higher frequency the electrons will have more kinetic energy. f. Below the threshold frequency, no matter how long you wait, no electrons will be emitted
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


