Question: Moving to another question will save this response. Question 4 For the following reaction, AH for the reaction is +206.1 kJ/mol, while AS is +215J/Komol
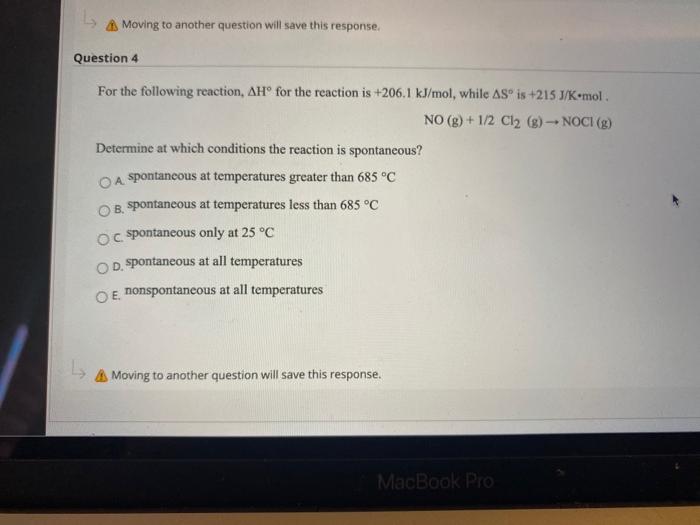
Moving to another question will save this response. Question 4 For the following reaction, AH for the reaction is +206.1 kJ/mol, while AS is +215J/Komol NO(g) + 1/2 Cl2 (g) - NOCI (g) Determine at which conditions the reaction is spontaneous? O A spontaneous at temperatures greater than 685 C B. spontaneous at temperatures less than 685 C oc spontaneous only at 25C OD. spontaneous at all temperatures O E nonspontaneous at all temperatures L Moving to another question will save this response. MacBook Pro
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


