Question: pls solve for part 4, got wrong miltiple times with answer listed. answer is not any form of 1.86. The freezing point of a 0.10m
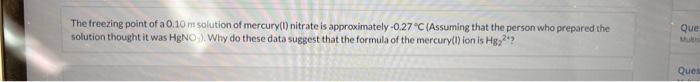
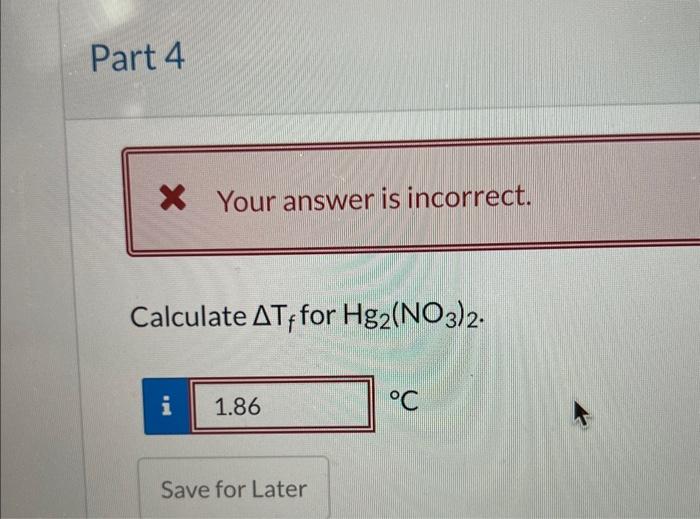
The freezing point of a 0.10m solution of mercury(1) nitrate is approximately- 0.27C (Assuming that the person who prepared the solution thought it was HgNO7). Why do these data suggest that the formula of the mercury(1) ion is Hg22+? ? x Your answer is incorrect. Calculate Tf for Hg2(NO3)2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


