Question: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which bond in the structure below would give rise
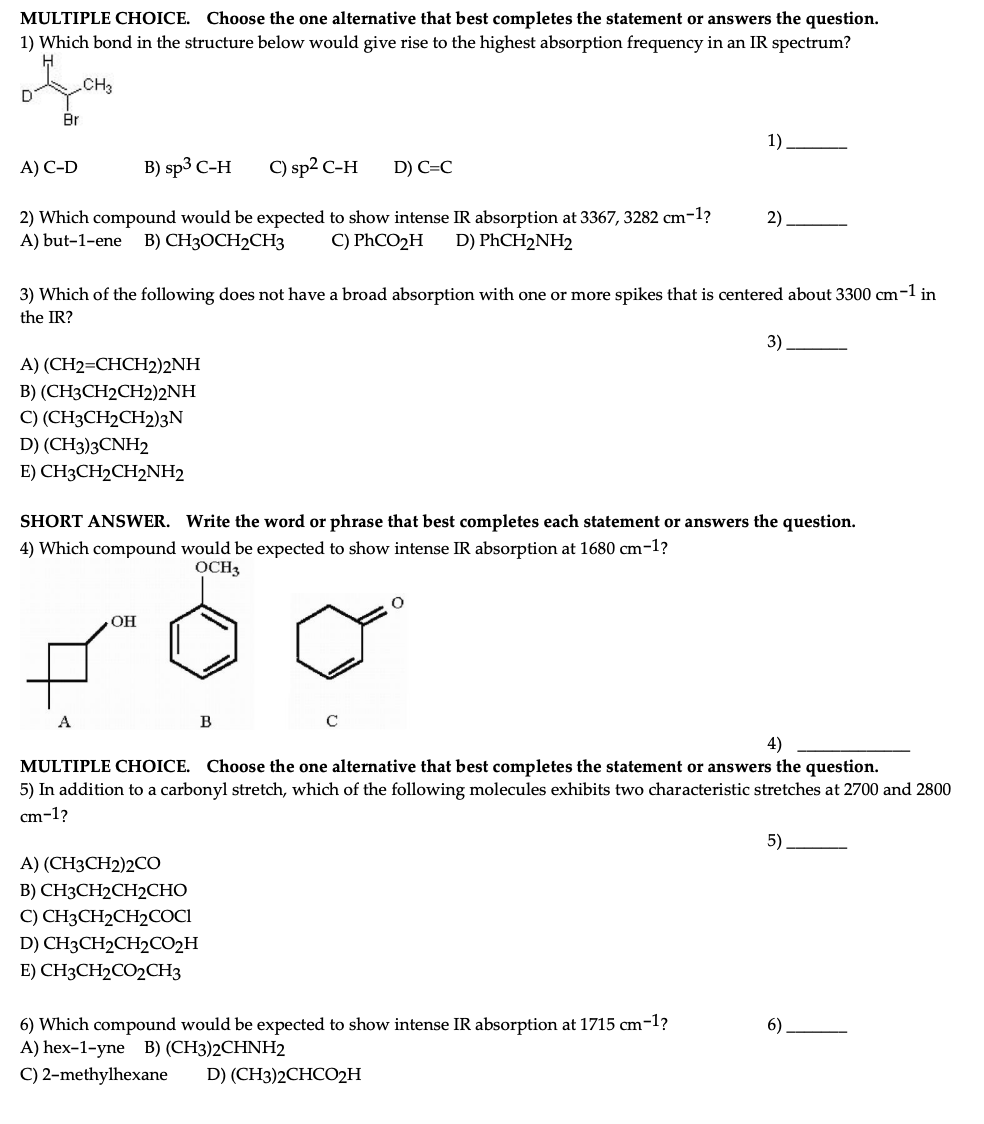
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which bond in the structure below would give rise to the highest absorption frequency in an IR spectrum? CH3 D Br 1) A) C-D B) sp3 C-H C) sp2 C-H D) C=C 2) 2) Which compound would be expected to show intense IR absorption at 3367, 3282 cm-1? A) but-1-ene B) CH3OCH2CH3 C) PhCO2H D) PhCH2NH2 3) Which of the following does not have a broad absorption with one or more spikes that is centered about 3300 cm-1 in the IR? 3) A) (CH2=CHCH2)2NH B) (CH3CH2CH2)2NH C) (CH3CH2CH2)3N D) (CH3)3CNH2 E) CH3CH2CH2NH2 SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. 4) Which compound would be expected to show intense IR absorption at 1680 cm-1? OCH3 OH B 4) MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 5) In addition to a carbonyl stretch, which of the following molecules exhibits two characteristic stretches at 2700 and 2800 cm-1? 5) A) (CH3CH2)2CO B) CH3CH2CH2CHO C) CH3CH2CH2COCI D) CH3CH2CH2C02H E) CH3CH2CO2CH3 6). 6) Which compound would be expected to show intense IR absorption at 1715 cm-1? A) hex-1-yne B) (CH3)2CHNH2 C)2-methylhexane D) (CH3)2CHCO2H
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


