Question: Multiple Choice: Circle the best answer: ( 2 pts each). 1. Which of the following choices contain the most de-shielded proton? A. (CH3)3CCH3 B. CH2CH2

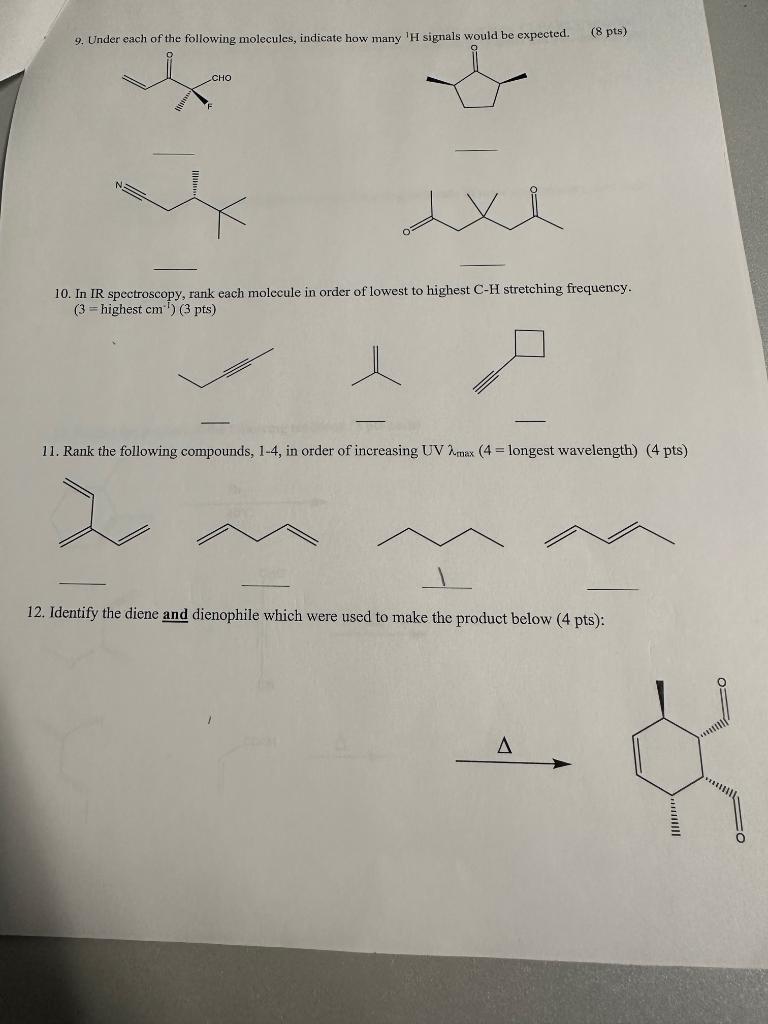
Multiple Choice: Circle the best answer: ( 2 pts each). 1. Which of the following choices contain the most de-shielded proton? A. (CH3)3CCH3 B. CH2CH2 C. CF3H D. (CH3)2CHCH3 E. CF2H2 2. What is the relative area under the peaks of a quartet? A. 1:1:1:1 B. 1:2:2:1 (C) 1:3:3:1 D. 1:4:4:1 E. 1:5:5:1 3. What species cannot be detected by a Mass-Spectrometer? A. Radical B. Cation C. Anion D. Radical Cation E. More than one of these 4. A chemist obtains a mass spectrum of an unknown compound and notices a profound M+2 of approximately 49% intensity. Which of the following compounds could this be? A. 3-chloropentane B. 2-bromohexane C. T-butyl alcohol D. Hexane E. 2-iodohexane 5. True or False - Considering carbon NMR: ( 3 pts). Carbon NMR will often split into multiplets A carboxylic acid carbon will have a chemical shift of 12ppm Carbon NMR utilizes 13C nuclei, whereas 12C nuclei are not observed 6. Draw any small molecule that you would expect to exhibit at least 1 sharp intense peak at 2250cm1 in an IR spectrum. ( 3 pts) CH3CH2CH2CO2H 7. For the following three dienes, label which would react the fastest in a Diels-Alder reaction, and label which would react the slowest in a D.A. reaction. (3 pts) 9. Under each of the following molecules, indicate how many 1H signals would be expected. (8pts) 10. In IR spectroscopy, rank each molecule in order of lowest to highest CH stretching frequency. (3= highest cm1)(3pts) 11. Rank the following compounds, 14, in order of increasing UV max(4= longest wavelength) ( 4 pts) 12. Identify the diene and dienophile which were used to make the product below ( 4 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


