Question: My Notes X Temperature = 20'C Pressure = 1 atm Boiling temperature = 100*C The law of thermal expansion for relative small intervals of temperatures
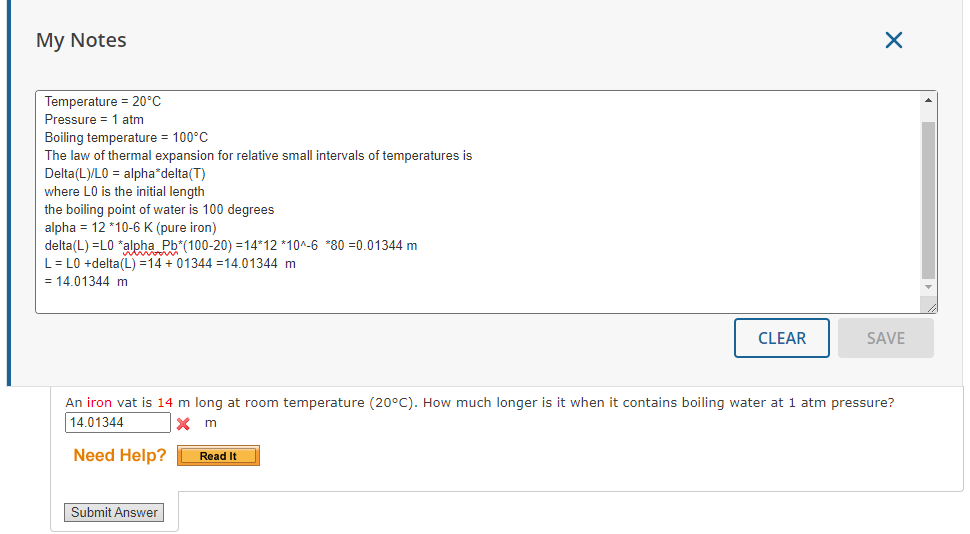
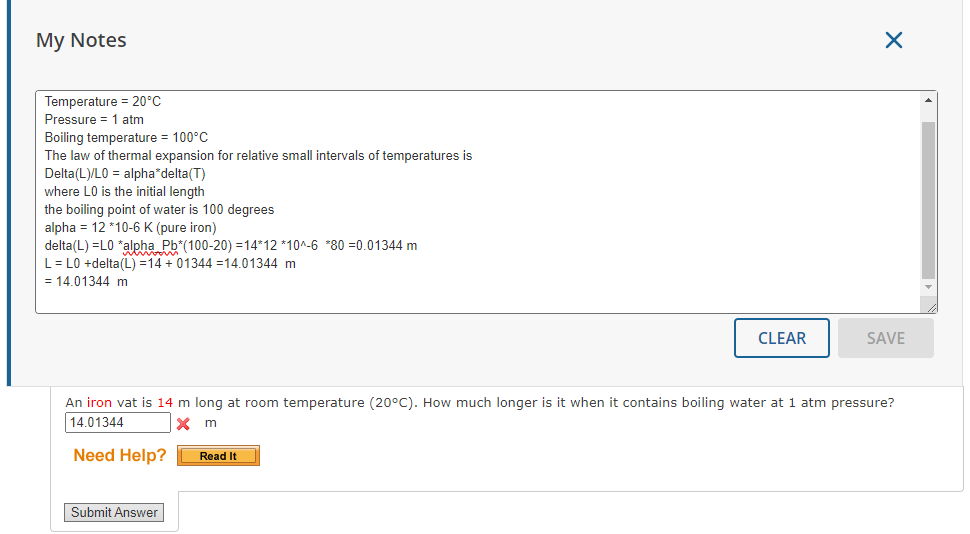
My Notes X Temperature = 20'C Pressure = 1 atm Boiling temperature = 100*C The law of thermal expansion for relative small intervals of temperatures is Delta(L)/LO = alpha*delta(T) where LO is the initial length the boiling point of water is 100 degrees alpha = 12 *10-6 K (pure iron) delta(L) =L0 *alpha Pb*(100-20) =14*12 *10^-6 *80 =0.01344 m L = LO +delta(L) =14 + 01344 =14.01344 m = 14.01344 m CLEAR SAVE An iron vat is 14 m long at room temperature (200C). How much longer is it when it contains boiling water at 1 atm pressure? 14.01344 X m Need Help? Read It Submit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


