Question: My PP = 422.3, this number should be between 123.4 and 987.6. My q = 2, this number should be between 1 and 9. Task

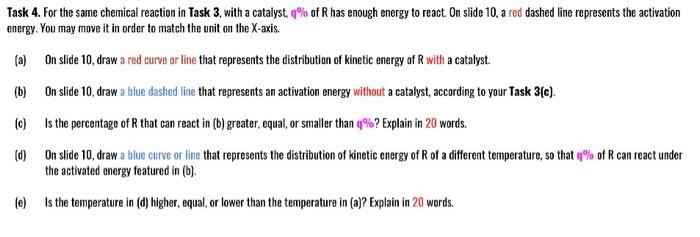
My PP = 422.3, this number should be between 123.4 and 987.6. My q = 2, this number should be between 1 and 9. Task 4. For the same chemical reaction in Task 3, with a catalyst, % of R has enough energy to react. On slide 10, a red dashed line represents the activation energy. You may move it in order to match the unit on the X-axis. (a) On slide 10, draw a red curve or line that represents the distribution of kinetic energy of R with a catalyst. (b) On slide 10, draw a blue dashed line that represents an activation energy without a catalyst, according to your Task 3(c). (c) Is the percentage of that can react in (b) greater, equal, or smaller than %? Explain in 20 words. (d) On slide 10, draw a blue curve or line that represents the distribution of kinetic energy of R of a different temperature, so that % of R can react under the activated energy featured in (b). (e) Is the temperature in (d) higher, equalor lower than the temperature in (a)? Explain in 20 words. My PP = 422.3, this number should be between 123.4 and 987.6. My q = 2, this number should be between 1 and 9. Task 4. For the same chemical reaction in Task 3, with a catalyst, % of R has enough energy to react. On slide 10, a red dashed line represents the activation energy. You may move it in order to match the unit on the X-axis. (a) On slide 10, draw a red curve or line that represents the distribution of kinetic energy of R with a catalyst. (b) On slide 10, draw a blue dashed line that represents an activation energy without a catalyst, according to your Task 3(c). (c) Is the percentage of that can react in (b) greater, equal, or smaller than %? Explain in 20 words. (d) On slide 10, draw a blue curve or line that represents the distribution of kinetic energy of R of a different temperature, so that % of R can react under the activated energy featured in (b). (e) Is the temperature in (d) higher, equalor lower than the temperature in (a)? Explain in 20 words
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


