Question: My question is, would it be possible to solve this by using the pH formula and getting the concentration of the (H + ) ions
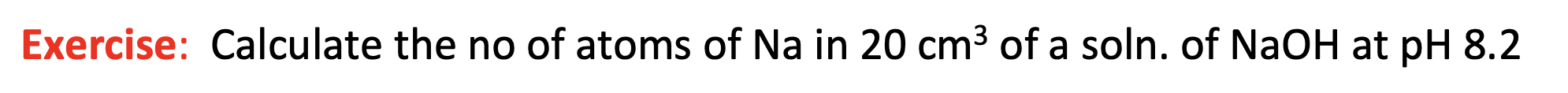
My question is, would it be possible to solve this by using the pH formula and getting the concentration of the (H+) ions in the solution. Then take that concentration and use it in the kw formula, which then gives you the (OH-) concentration.
If that is true, could you please work it out for me, as i think i keep getting my working out wrong somewhere?
Thank you :)
Exercise: Calculate the no of atoms of Na in 20cm3 of a soln. of NaOH at pH 8.2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


