Question: n One of the proposed ways to obtain higher efficiency from fuels in vehicles and avoid pollutants (and carbon dioxide) is by the use of
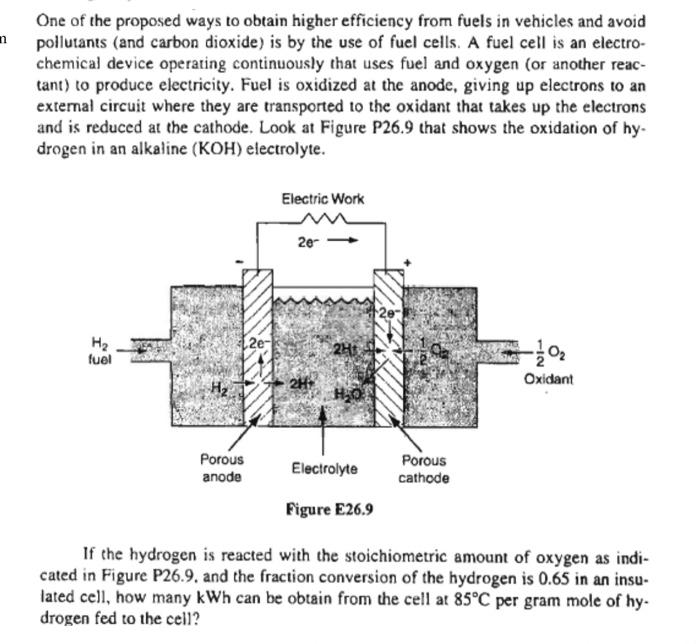
n One of the proposed ways to obtain higher efficiency from fuels in vehicles and avoid pollutants (and carbon dioxide) is by the use of fuel cells. A fuel cell is an electro- chemical device operating continuously that uses fuel and oxygen (or another reac- tant) to produce electricity. Fuel is oxidized at the anode, giving up electrons to an external circuit where they are transported to the oxidant that takes up the electrons and is reduced at the cathode. Look at Figure P26.9 that shows the oxidation of hy. drogen in an alkaline (KOH) electrolyte. Electric Work 2e- 12e- Hz 2e- 25 fuel NI Oxidant Porous anode Electrolyte Porous cathode Figure E26.9 If the hydrogen is reacted with the stoichiometric amount of oxygen as indi- cated in Figure P26.9. and the fraction conversion of the hydrogen is 0.65 in an insu- lated cell, how many kWh can be obtain from the cell at 85C per gram mole of hy. drogen fed to the cell? n One of the proposed ways to obtain higher efficiency from fuels in vehicles and avoid pollutants (and carbon dioxide) is by the use of fuel cells. A fuel cell is an electro- chemical device operating continuously that uses fuel and oxygen (or another reac- tant) to produce electricity. Fuel is oxidized at the anode, giving up electrons to an external circuit where they are transported to the oxidant that takes up the electrons and is reduced at the cathode. Look at Figure P26.9 that shows the oxidation of hy. drogen in an alkaline (KOH) electrolyte. Electric Work 2e- 12e- Hz 2e- 25 fuel NI Oxidant Porous anode Electrolyte Porous cathode Figure E26.9 If the hydrogen is reacted with the stoichiometric amount of oxygen as indi- cated in Figure P26.9. and the fraction conversion of the hydrogen is 0.65 in an insu- lated cell, how many kWh can be obtain from the cell at 85C per gram mole of hy. drogen fed to the cell
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


