Question: Name: Data Table: Table 1. Mass data for magnesium oxide combustion reaction. Mass of Mass of Mass of crucible, lid, crucible. lid, Mass after 2
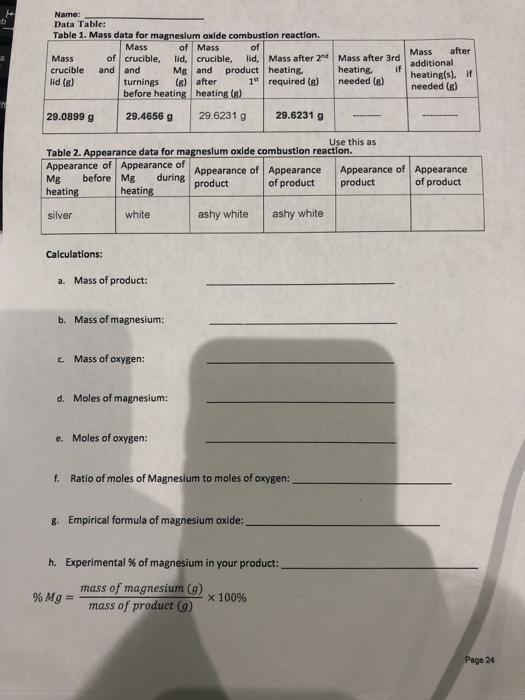

Name: Data Table: Table 1. Mass data for magnesium oxide combustion reaction. Mass of Mass of Mass of crucible, lid, crucible. lid, Mass after 2 Mass after 3rd crucible and and Mg and product heating, heating, if lid (8) turnings (s) after 19 required (8) needed (e) before heating heating (8) Mass after additional heating(s). needed (8) 29.0899 g 29.4656 g 29.6231 g 29.6231 g Use this as Table 2. Appearance data for magnesium oxide combustion reaction. Appearance of Appearance of Appearance of Appearance Appearance of Appearance Mg before Mg during product of product product of product heating heating silver white ashy white ashy white Calculations: a. Mass of product: b. Mass of magnesium Mass of oxygen: d. Moles of magnesium e. Moles of oxygen: E. Ratio of moles of Magnesium to moles of oxygen: 8. Empirical formula of magnesium oxide: h. Experimental % of magnesium in your product: mass of magnesium (9) % Mg = X 100% mass of product (9) Page 24 Name: Actual formula of magnesium oxide (based on charge of Mg and O): Theoretical mass % of Mg based on the actual formula of magnesium oxide. (Hint: you will need the molar mass of magnesium and magnesium oxide). k. Percent error in % Mg: experimental value theoretical value % error = x 100% theoretical value Discussion questions 1. Write the balanced chemical equation for the formation of magnesium oxide. 2 Mg (5) +0:19) - 2 Ingo (s) 2. Magnesium nitride is an impurity formed by the reaction of magnesium with nitrogen. Write down the balanced chemical equation for the formation of magnesium nitride. 3. What happens when we add water to magnesium nitride? Write down the balanced chemical equation. Hint: one of the product is ammonia: (NH) 4. Discuss two sources of error in this experiment that might account for a high percentage error in % Mg Page 25
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


