Question: need help on second page DATA: LABORATORY 5 OBSERVATIONS Anything you observed, condensation, steam, smoke, spilled compound, etc. EMPIRICAL FORMULA OF MAGNESIUM OXIDE COMPOUND INDIVIDUAL
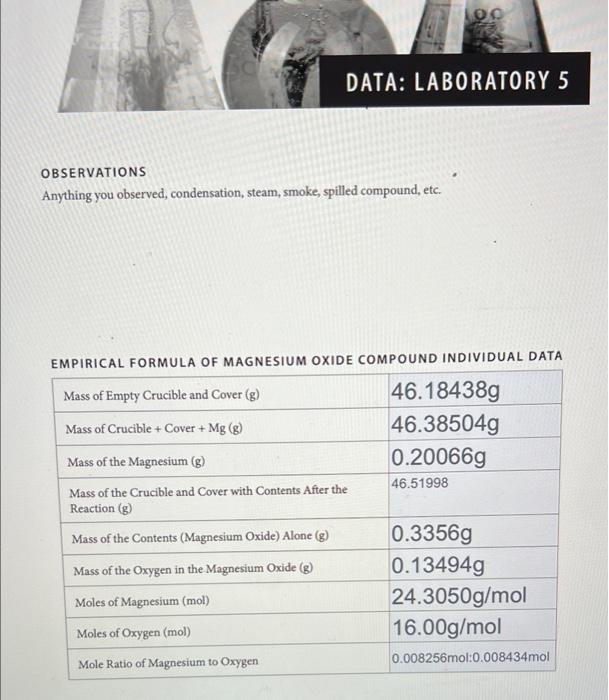
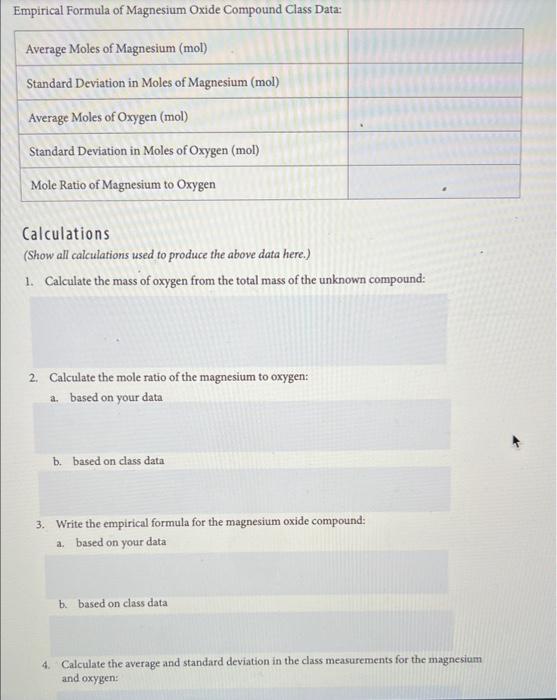
DATA: LABORATORY 5 OBSERVATIONS Anything you observed, condensation, steam, smoke, spilled compound, etc. EMPIRICAL FORMULA OF MAGNESIUM OXIDE COMPOUND INDIVIDUAL DATA Mass of Empty Crucible and Cover (g) 46.18438g Mass of Crucible + Cover + Mg (g) 46.38504g Mass of the Magnesium (g) 0.200669 46.51998 Mass of the Crucible and Cover with Contents After the Reaction (g) Mass of the Contents (Magnesium Oxide) Alone (g) 0.3356g Mass of the Oxygen in the Magnesium Oxide (8) 0.13494g Moles of Magnesium (mol) 24.3050g/mol Moles of Oxygen (mol) 16.00g/mol Mole Ratio of Magnesium to Oxygen 0.008256mol:0,008434mol Empirical Formula of Magnesium Oxide Compound Class Data: Average Moles of Magnesium (mol) Standard Deviation in Moles of Magnesium (mol) Average Moles of Oxygen (mol) Standard Deviation in Moles of Oxygen (mol) Mole Ratio of Magnesium to Oxygen Calculations (Show all calculations used to produce the above data here.) 1. Calculate the mass of oxygen from the total mass of the unknown compound: 2. Calculate the mole ratio of the magnesium to oxygen: a. based on your data b. based on class data 3. Write the empirical formula for the magnesium oxide compound: a. based on your data b. based on class data 4. Calculate the average and standard deviation in the class measurements for the magnesium and oxygen
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


