Question: NaOH concentration: 0.15M data table question (pls answer step by step) 10 ml of acetic acid TRIAL 1 Volume NaOH PH (mL) 3 Oml 4
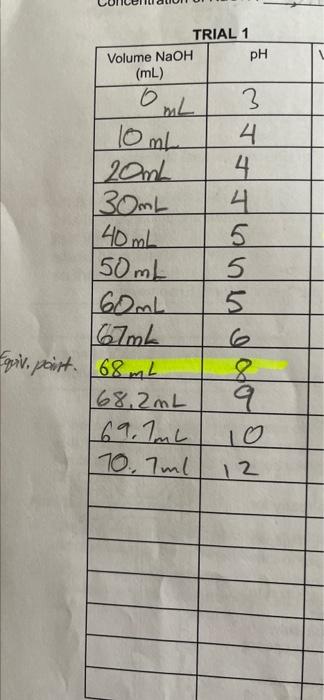

TRIAL 1 Volume NaOH PH (mL) 3 Oml 4 10mL 20mL 130mL 40mL 150mL 160mL 167ml Equiv, point. [68 mL 168.2mL 69.1mL 10.7ml 4 5 5 5 Moond goo 9 10 12 Find % lonization and K 4. Use the pH of the acetic acid before adding water to calculate the [H,O*). Use this and the concentration of the acid calculated to find the % ionization of acetic acid. tont of anotin lothonninl onid in mentor
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


