Question: Natural gas, whose composition and properties are given in Table 3, is fed to the boiler at the desired flow rate under normal conditions. Assuming
Natural gas, whose composition and properties are given in Table 3, is fed to the boiler at the desired flow rate under normal conditions. Assuming that there is complete combustion, calculate the following in case the natural gas is burned with excess air, according to the data given in Table 4. a. the required amount of air, b. The amount and composition of the produced flue gas, c. Calculate the flue gas composition on a dry basis. D. Calculate the heat released by combustion.
table 4 (Natural gas flow rate (kmol/h) =280,00 Excess Air (%)70
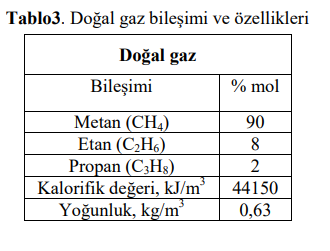
Tablo3. Doal gaz bileimi ve zellikleri \begin{tabular}{|c|c|} \hline \multicolumn{2}{|c|}{ Doal gaz } \\ \hline Bileimi & %mol \\ \hline Metan (CH4) & 90 \\ \hline Etan (C2H6) & 8 \\ \hline Propan (C3H8) & 2 \\ \hline Kalorifik deeri, kJ/m3 & 44150 \\ \hline Younluk, kg/m3 & 0,63 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


