Question: Need a detailed answer and graphical method Compound A reacts with B according to the reaction A+Bk2B,k=1molminlt The reaction is elementary. For a feed containing
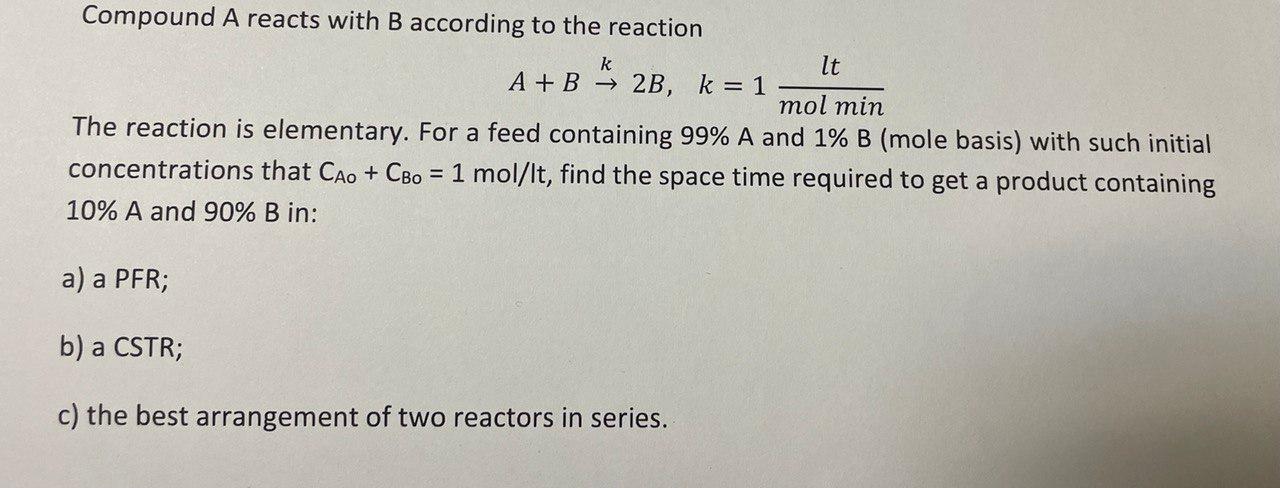 Need a detailed answer and graphical method
Need a detailed answer and graphical method
Compound A reacts with B according to the reaction A+Bk2B,k=1molminlt The reaction is elementary. For a feed containing 99%A and 1%B (mole basis) with such initial concentrations that CAO+CBO=1mol/lt, find the space time required to get a product containing 10%A and 90% B in: a) a PFR; b) a CSTR; c) the best arrangement of two reactors in series
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


