Question: need a help with this lap report QUESTION 1. State the stoichiometric relationship of sodium thiosulfate moles that are equivalent to one mole of sodium
need a help with this lap report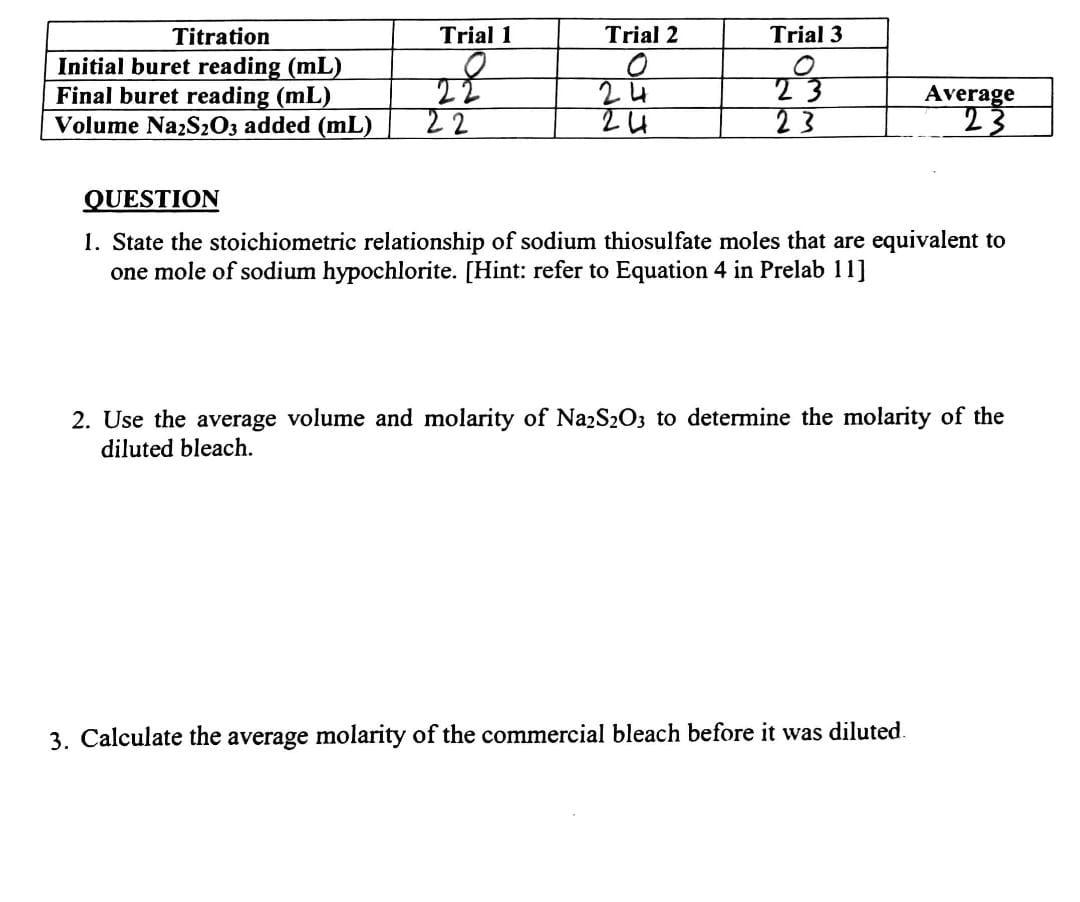
QUESTION 1. State the stoichiometric relationship of sodium thiosulfate moles that are equivalent to one mole of sodium hypochlorite. [Hint: refer to Equation 4 in Prelab 11] 2. Use the average volume and molarity of Na2S2O3 to determine the molarity of the diluted bleach. 3. Calculate the average molarity of the commercial bleach before it was diluted
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


