Question: need answer for all 4 :) Use the following information for questions 45 : Succinic acid is a substance produced by lichens. Chemical analysis indicates

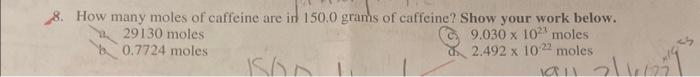

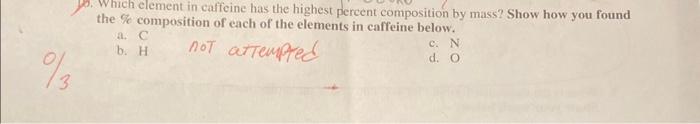
Use the following information for questions 45 : Succinic acid is a substance produced by lichens. Chemical analysis indicates it is composed of 40.68% carbon, 5.08% hydrogen, and the rest oxygen. How did you calculage empirical Formula? 4. What is the empirical formula for succinic acid? For any credit, show ALL of your work below! 8. How many moles of caffeine are in 150.0 grams of caffeine? Show your work below. a 29130 moles b 0.7724 moles (cy 9.0301023 moles 2.4921022 moles What is the mass, in grams, of 7.801024 molecules of caffeine? Show your work below. a.) 13.0 grams b. 4.701048 grams 7,86.01024 modoules mol a.2.421046gramsYousrapedroo2520grams Which element in caffeine has the highest percent composition by mass? Show how you found the % composition of each of the elements in caffeine below. a. C b. H not atremprec c. N d. O
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


