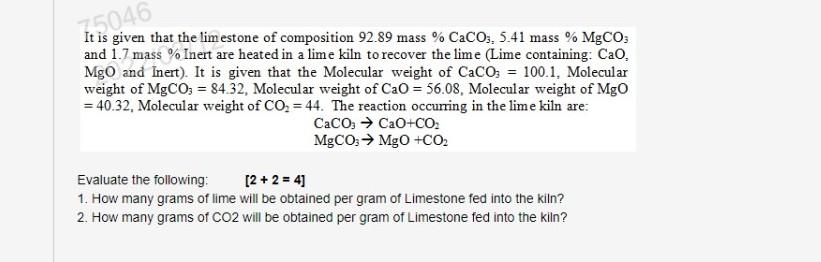
Question: need answer with steps 75046 It is given that the limestone of composition 92.89 mass % CaCO3, 5.41 mass % MgCO: and 1.7 mass %
need answer with steps
75046 It is given that the limestone of composition 92.89 mass % CaCO3, 5.41 mass % MgCO: and 1.7 mass % Inert are heated in a lime kiln to recover the lime (Lime containing: Ca. MgO and Inert). It is given that the Molecular weight of CaCO3 = 100.1, Molecular weight of MgCO3 = 84.32. Molecular weight of Cao = 56.08, Molecular weight of Mgo = 40.32, Molecular weight of CO2 = 44. The reaction occurring in the lime kiln are: CaCO3 + CaO+CO: MgCO: MgO +CO Evaluate the following: [2 + 2 = 4) 1. How many grams of lime will be obtained per gram of Limestone fed into the kiln? 2. How many grams of CO2 will be obtained per gram of Limestone fed into the kiln
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


