Question: Data Sheet Part A: Dependence of reaction rate on Concentration Table 1 [1] [Bro] [H] Tamp. (C) 22.9 C reaction nix Time (5) 1
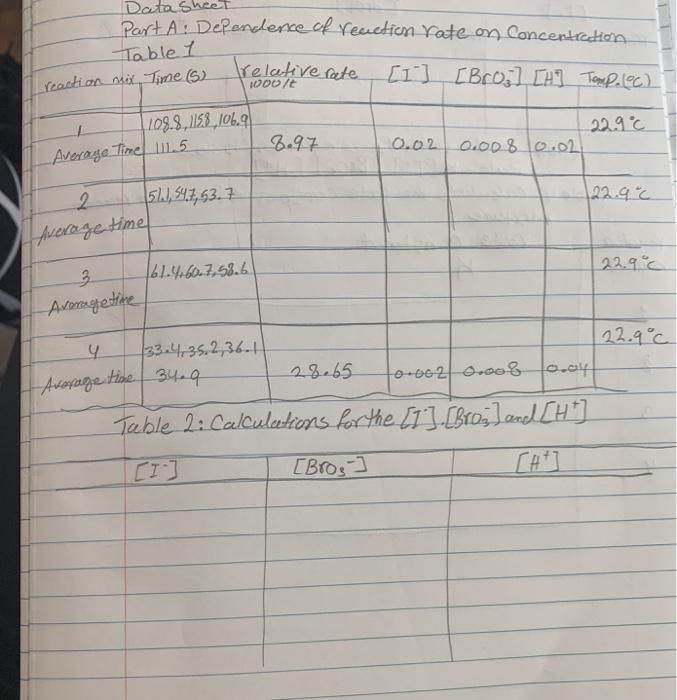
![[1] [Bro] [H] Tamp. (C) 22.9 C reaction nix Time (5) 1](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2020/07/5f1b47d8cd83b_image20200725T021139.680.png)
Data Sheet Part A: Dependence of reaction rate on Concentration Table 1 [1] [Bro] [H] Tamp. (C) 22.9 C reaction nix Time (5) 1 Average Time 111.5 108.8,1158, 106.9 2 Average time 3 Average time 511, 547, 53.7 relative rate woo/t 61.4.60.7.58.6 4 Average time 34.9 33.435,2,36.1 8.97 28.65 0.02 0.008 0.02 22.9 c 0.002 0.008 0.ay Table 2: Calculations for the [1]. [Bros] and [HT] [1] [Bros] 22.9C 22.9C m= 1 reaction mixture 2 3 Table 3: Calculate the effect (min.P) [1-1 [Broj] 4 Complete the rate law equation below. Rede= K[I] [Bros ] [H+] Table 4. Culculate the rate constant K for each reaction mixture rate Constant K (H+) Em
Step by Step Solution
3.44 Rating (167 Votes )
There are 3 Steps involved in it
Solution reaction 1 Average Bezage 4 Table1 arix Time 9 relative rate 5 Bros ... View full answer

Get step-by-step solutions from verified subject matter experts


