Question: Need help answering 5,6, and 7 Calculating Reactant Orders and Determining the Rate Law 5. Calculate the initial concentration of Iand S2O82 for each experiment.
Need help answering 5,6, and 7
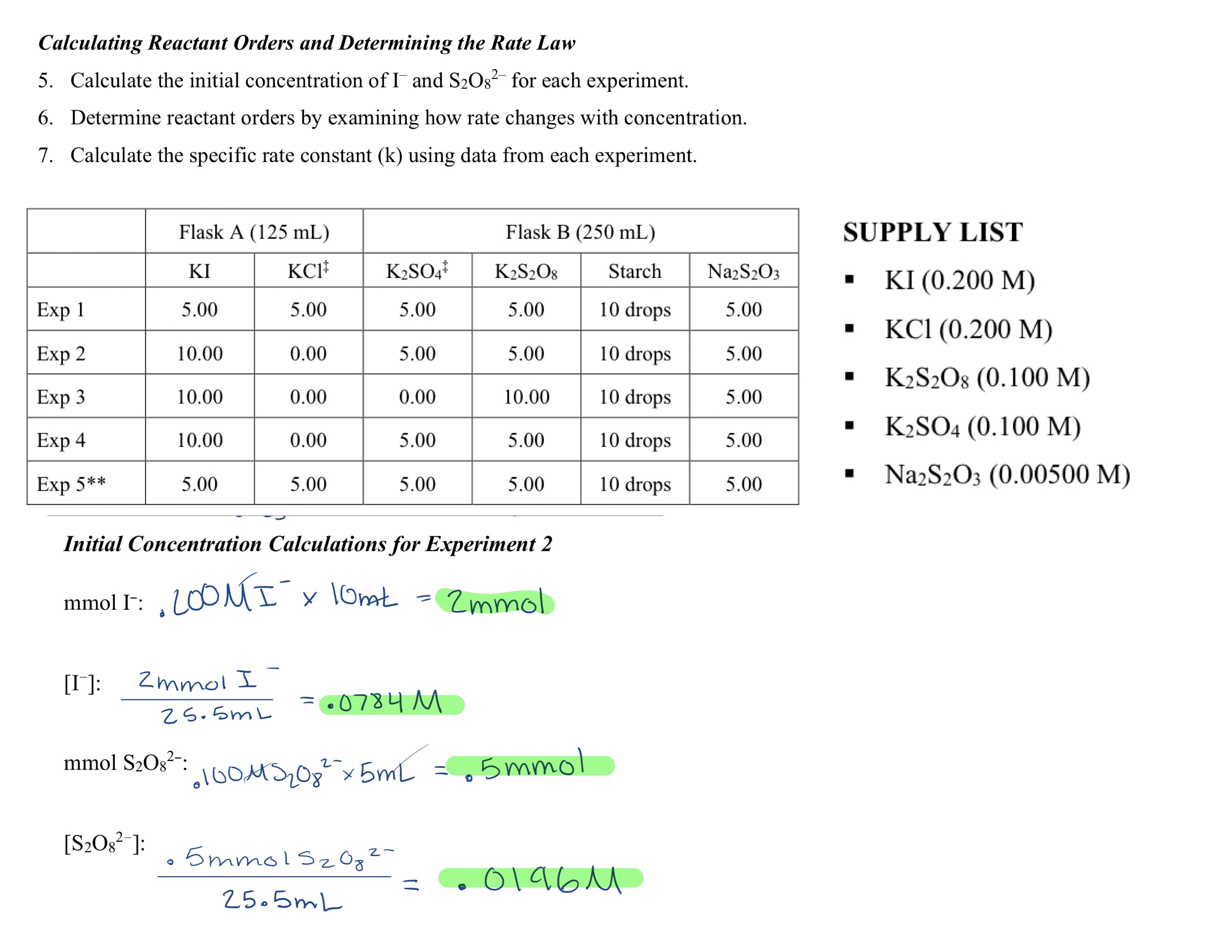
Calculating Reactant Orders and Determining the Rate Law 5. Calculate the initial concentration of Iand S2O82 for each experiment. 6. Determine reactant orders by examining how rate changes with concentration. 7. Calculate the specific rate constant (k) using data from each experiment. SUPPLY LIST - KI(0.200M) - KCl(0.200M) - K2S2O8(0.100M) - K2SO4(0.100M) - Na2S2O3(0.00500M) Initial Concentration Calculations for Experiment 2 mmolI:.200MI10nat=2mmol [I]:25.5mL2mmolI=.0784M mmolSO82:0100MS2O825mL=.5mmol [S2O82]:25.5mL.5mmolS2O82=.0196M Calculating Reactant Orders and Determining the Rate Law 5. Calculate the initial concentration of Iand S2O82 for each experiment. 6. Determine reactant orders by examining how rate changes with concentration. 7. Calculate the specific rate constant (k) using data from each experiment. SUPPLY LIST - KI(0.200M) - KCl(0.200M) - K2S2O8(0.100M) - K2SO4(0.100M) - Na2S2O3(0.00500M) Initial Concentration Calculations for Experiment 2 mmolI:.200MI10nat=2mmol [I]:25.5mL2mmolI=.0784M mmolSO82:0100MS2O825mL=.5mmol [S2O82]:25.5mL.5mmolS2O82=.0196M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


