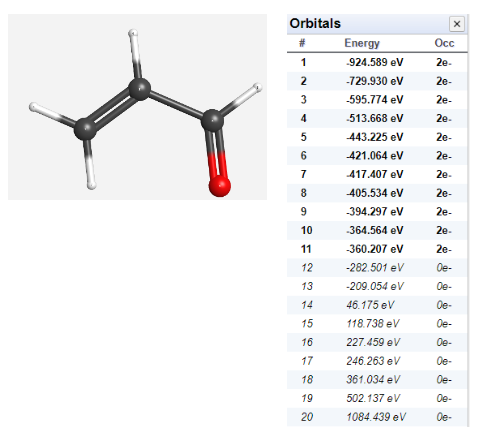
Question: Need help Don't really understand LUMO and HOMO begin{tabular}{cll} Orbitals & hline# & Energy & Occ hline 1 & 924.589eV & 2e

Need help Don't really understand LUMO and HOMO
\begin{tabular}{cll} Orbitals & \\ \hline# & Energy & Occ \\ \hline 1 & 924.589eV & 2e \\ 2 & 729.930eV & 2e \\ 3 & 595.774eV & 2e \\ 4 & 513.668eV & 2e \\ 5 & 443.225eV & 2e \\ 6 & 421.064eV & 2e \\ 7 & 417.407eV & 2e \\ 8 & 405.534eV & 2e \\ 9 & 394.297eV & 2e \\ 10 & 364.564eV & 2e \\ 11 & 360.207eV & 2e \\ 12 & 282.501eV & 0e \\ 13 & 209.054eV & 0e \\ 14 & 46.175eV & 0e \\ 15 & 118.738eV & 0e \\ 16 & 227.459eV & 0e \\ 17 & 246.263eV & 0e \\ 18 & 361.034eV & 0e \\ 19 & 502.137eV & 0e \\ 20 & 1084.439eV & 0e \end{tabular} Rotate the molecule and sketch (or print screen) the MOs for the HOMO and the LUMO, by selecting the gray box. Label all nodes and regions of highest and lowest electron density for both orbitals. \begin{tabular}{cll} Orbitals & \\ \hline# & Energy & Occ \\ \hline 1 & 924.589eV & 2e \\ 2 & 729.930eV & 2e \\ 3 & 595.774eV & 2e \\ 4 & 513.668eV & 2e \\ 5 & 443.225eV & 2e \\ 6 & 421.064eV & 2e \\ 7 & 417.407eV & 2e \\ 8 & 405.534eV & 2e \\ 9 & 394.297eV & 2e \\ 10 & 364.564eV & 2e \\ 11 & 360.207eV & 2e \\ 12 & 282.501eV & 0e \\ 13 & 209.054eV & 0e \\ 14 & 46.175eV & 0e \\ 15 & 118.738eV & 0e \\ 16 & 227.459eV & 0e \\ 17 & 246.263eV & 0e \\ 18 & 361.034eV & 0e \\ 19 & 502.137eV & 0e \\ 20 & 1084.439eV & 0e \end{tabular} Rotate the molecule and sketch (or print screen) the MOs for the HOMO and the LUMO, by selecting the gray box. Label all nodes and regions of highest and lowest electron density for both orbitals
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


