Question: need help finding the cooling time (Tb0=95 & Tw=25, Tb is the temperature of the problem) if Tb was 45 Tb-Tw= 45-25 & Tb0-Tw=95-25. Aniron
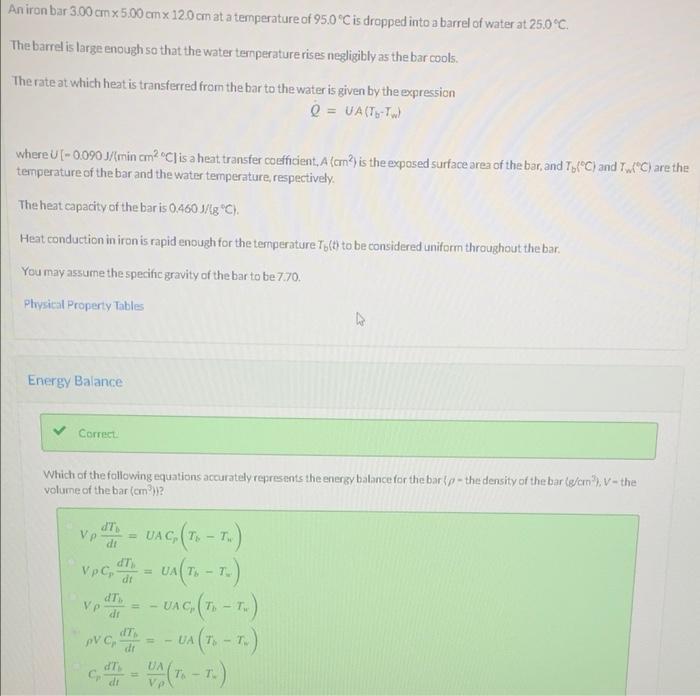

Aniron bar 3.00 cm x 5.00 cm x 120 cm at a temperature of 95.0 C is dropped into a barrel of water at 25.0C. The barrel is large enough so that the water temperaturerises negligibly as the bar cools. The rate at which heat is transferred from the bar to the water is given by the expression Q = UA(T-TW where U (- 0.0903/min cm2 C] is a heat transfer coefficient, A (cm?) is the exposed surface area of the bar, and TyfC) and C) are the temperature of the bar and the water temperature respectively. The heat capacity of the bar is 0.460 1/8 C). Heat conduction in iron is rapid enough for the temperature Ty(t) to be considered uniform throughout the bar You may assume the specific gravity of the bar to be 770. Physical Property Tables Energy Balance Correct Which of the following equations accurately represents the energy balance for the barp-the density of the bar (g/cm), V-the volume of the bar (cm) IT di dij - -T , dt Vp UAC( - ) ,T. . : vec d = UA (T6 - T.) - UAG (T. -T.) pvc, "I = - UA(1. - 1) Ve dT di dT , 7 dT Cooling Time Integrate the energy balance, apply the appropriate initial condition and solve for the time required for the bar to cool to 400C mnin
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


