Question: need help finding the rate law please A possible mechanism for the overall reaction Br2(g)+2NO(g)2NOBr(g) is NO(g)+Br2(g)k12k1NOBr2(g)NOBr2(g)+NO(g)k22NOBr(slow)(fast) Determine the rate law for this mechanism. Show
need help finding the rate law please 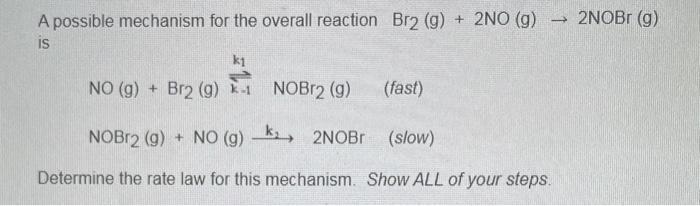

A possible mechanism for the overall reaction Br2(g)+2NO(g)2NOBr(g) is NO(g)+Br2(g)k12k1NOBr2(g)NOBr2(g)+NO(g)k22NOBr(slow)(fast) Determine the rate law for this mechanism. Show ALL of your steps
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


