Question: Need help identifying structures on IR and NMR graph. Lab 7 - NaBH4 Reduction of Vanillin CH3CH2OHNaBH4 VanillyI Alcohol Vanillin Molecular Formula: C8H3O3 MolecularFormula, CaH10O3

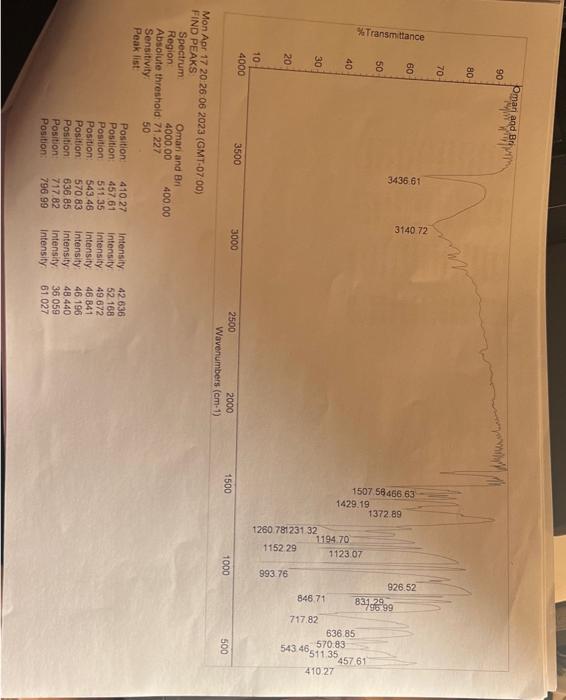
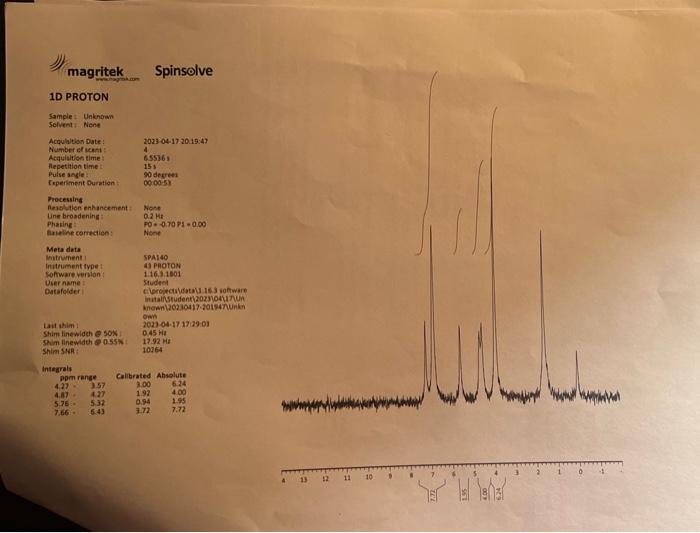
Lab 7 - NaBH4 Reduction of Vanillin CH3CH2OHNaBH4 VanillyI Alcohol Vanillin Molecular Formula: C8H3O3 MolecularFormula, CaH10O3 Molecular Weight: 152.15 Molecular Weight: 154,16 Introduction: Vanillin (4-hydroxy-3-methoxybenzaldehyde), is a pleasant smelling aromatic compound found in the pods of the vanilla plant (varilla planifolia). Vanillin is used widely as a flavoring additive for beverages, cooking, and as an aromatic additive for candles, potpourri, fragrances and air fresheners. It is also used as a starting materinl for the synthesis of such drugs as L-dopa, which is used for treating Parkincon's disease. In this lab, vanillin will be the starting material for the production of vanillyl alcohol, a compound which can serve as a valuable intermediate in the production of novel flavorings, perfumes and as a renewable starting material for the synthesis of biologically active molecules, A common method for preparing alcohols is the reduction of aldehydes to form 10 alcohols or the reduction of ketones to produce 2 alcohols. Metal hydrides of the Group III elements such as lithium aluminum hydride (LiAlH4) and sodium borohydride (NaBH4) are widely used to accomplish this task. Both reagents are ready sources of the hydride ion ( H:), a very strong base and when associated with aluminum or boron, a good nucleophile. Unfortunately, LiAlH is extremely reactive and can reduce any functional group that has a C=O,C=N bond and nitriles. On the other hand, NaBH4 is a much milder reducing agent, working effectively only with aldehydes, ketones and imines. In the present experiment, sodium borohydride is used because it is easily handled and the results of reductions using either of the two reagents are essentially the same. Materials Needed hot plate ring stand magnetic stirring bar 50mL Erlenmeyer flask, filter flask Buchner funnel filter paper clamp pH paper glass pipet rubberbulb Chemicals Needed vanillin NaBH4 ethanol 1MNaOH6MHCl
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


