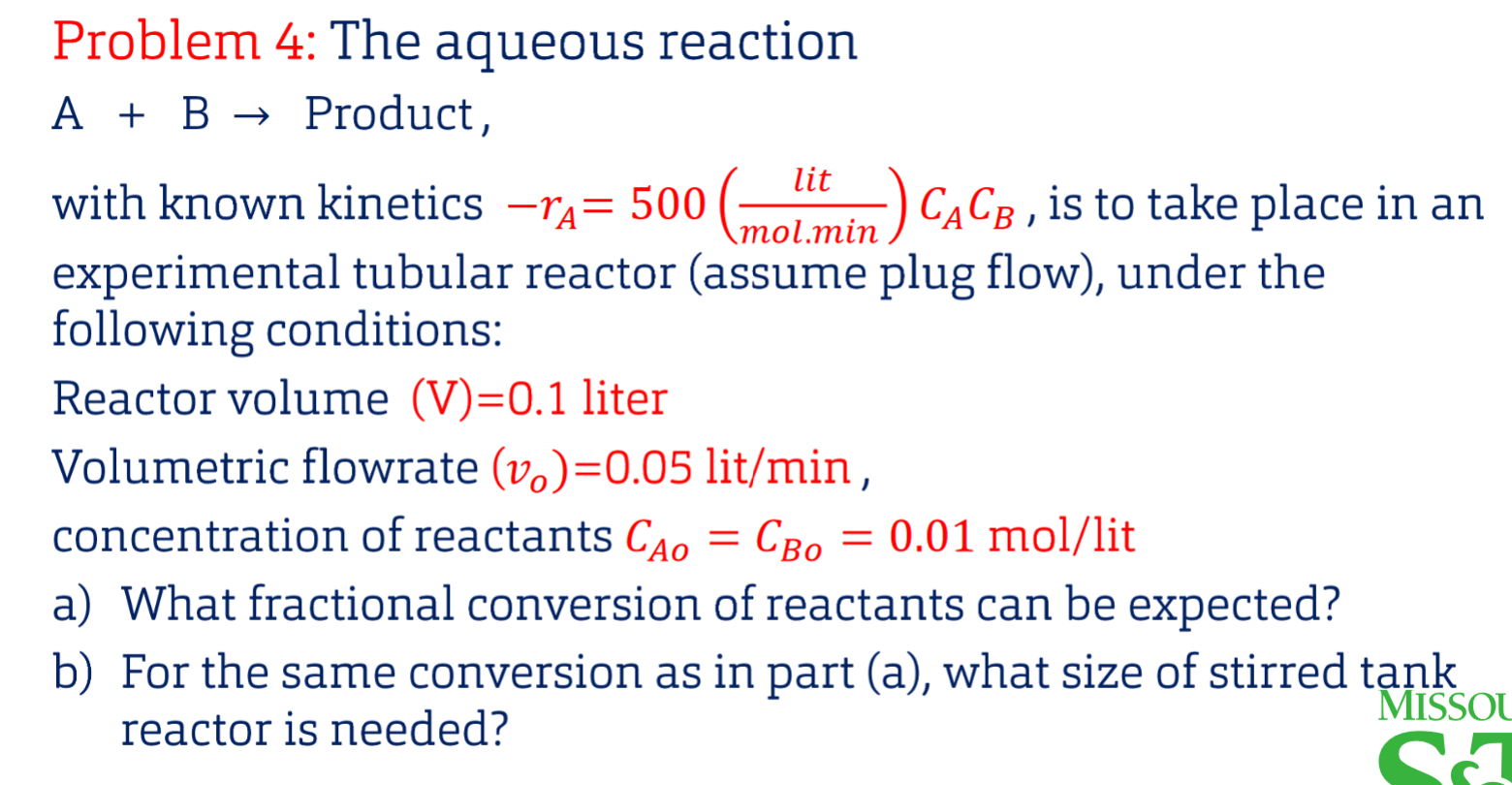
Question: Need Help on Part C and Part D pleaseeee. Thank you!! Problem 4: The aqueous reaction A+B Product, with known kinetics rA=500(mol.minlit)CACB, is to take
 Need Help on Part C and Part D pleaseeee. Thank you!!
Need Help on Part C and Part D pleaseeee. Thank you!!
Problem 4: The aqueous reaction A+B Product, with known kinetics rA=500(mol.minlit)CACB, is to take place in an experimental tubular reactor (assume plug flow), under the following conditions: Reactor volume (V)=0.1 liter Volumetric flowrate (vo)=0.05lit/min, concentration of reactants CAO=CBo=0.01mol/lit a) What fractional conversion of reactants can be expected? b) For the same conversion as in part (a), what size of stirred tank reactor is needed? Problem 4: c) What conversion can be expected in a mixed reactor equal in size to the plug flow reactor d) If the feed in (a) is altered such that CBo=0.015mol/lit,CBO=0.01 mol/lit, find XA
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


