Question: Problem 4: The aqueous reaction ( In mivin y ) Forite 4,12,11:595 A+B Product, with known kinetics rA=500(mol.mintit)CACB, is to take place in an test
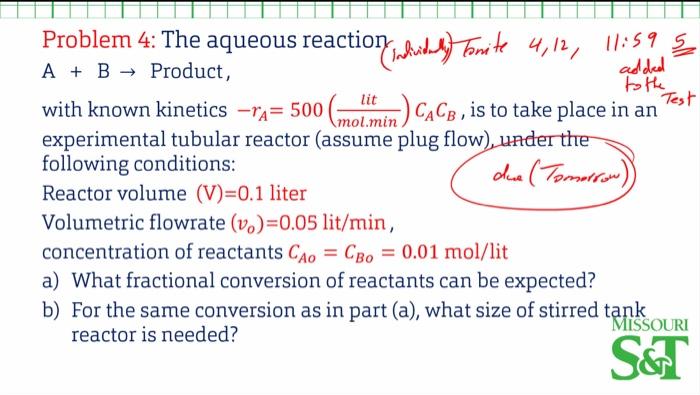
Problem 4: The aqueous reaction ( In mivin y ) Forite 4,12,11:595 A+B Product, with known kinetics rA=500(mol.mintit)CACB, is to take place in an test experimental tubular reactor (assume plug flow) under the following conditions: Reactor volume (V)=0.1 liter dua (Tomorrow)) Volumetric flowrate (vo)=0.05lit/min, concentration of reactants CAo=CBo=0.01mol/lit a) What fractional conversion of reactants can be expected? b) For the same conversion as in part (a), what size of stirred tank reactor is needed
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


