Question: Need help show work Activity 1: Discovering the Fundamental Unit of Charge There are many, many electrons with in a solid. Think about the number
Need help show work
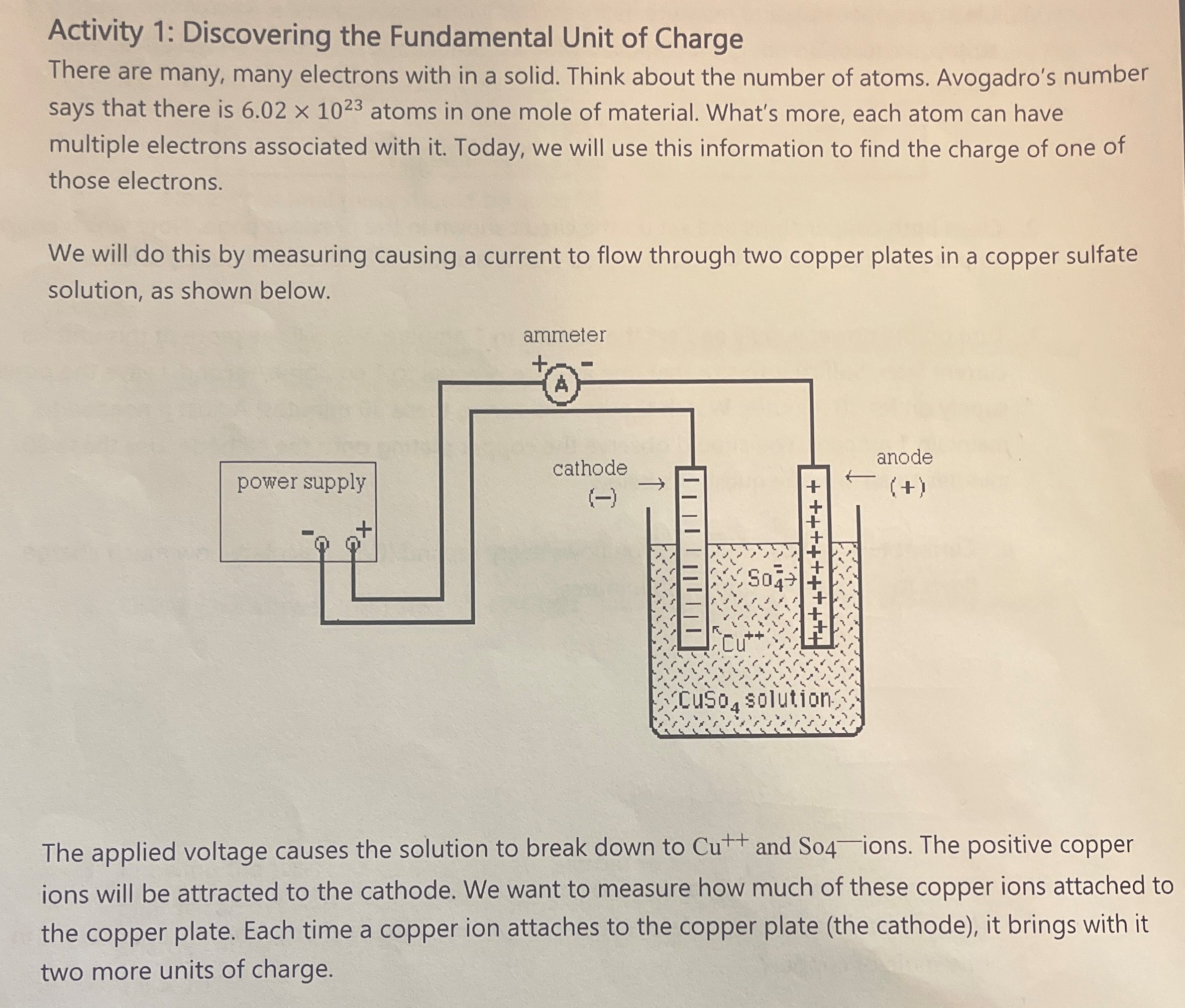



Activity 1: Discovering the Fundamental Unit of Charge There are many, many electrons with in a solid. Think about the number of atoms. Avogadro's number says that there is 6.02 x 1023 atoms in one mole of material. What's more, each atom can have multiple electrons associated with it. Today, we will use this information to find the charge of one of those electrons. We will do this by measuring causing a current to flow through two copper plates in a copper sulfate solution, as shown below. ammeter cathode anode power supply (+ ) cuso, solution The applied voltage causes the solution to break down to Cut and So4-ions. The positive copper ions will be attracted to the cathode. We want to measure how much of these copper ions attached to the copper plate. Each time a copper ion attaches to the copper plate (the cathode), it brings with it two more units of charge.1. Clean a copper strip and measure its mass. This will be your cathode. Remember which copp strip you chose! Do not get it confused with the other copper strip which will act as your anode. Mi = 14.038g 2. Clean both copper strips and set up the circuit shown in the previous page. Note which copper strip is your cathode. Fill the glass cup so that it is about 2/3 filled with CuSo4 solution. 3. Turn on the power supply and set the current to 1 ampere. We will see more of this unit for current later. Suffice it to say, that one ampere is equal to 1 coulomb/second. Leave the power supply on for 30 minutes. Watch the current during these 30 minutes! Adjust if needed to maintain 1 ampere. You should observe the copper plating onto the cathode. Use these 30 minutes to answer the questions below. a. Current tells us how much charge flows every second (C/s). Calculate how much charge flows to the copper strip in 30 minutes. b. Each copper ion (Cut+ ) carries two charge carriers with it. There are 6.02 x 1023 (NA) copper ions in one mole of substance (n). How many charge carriers are present in one mole of copper?4. Once your timer reaches 30 minutes, turn off the power supply immediately. Carefully remove and dry the cathode without disturbing the copper film formed on the strip. Measure the dry cathode and enter all information in the table below. Current (C/s ) Time (s) Initial Mass Mi Final Mass Mr 1200% 14,03 89 19.512 9 Note: Your final mass should be larger than your initial mass Analysis 1. Use the difference in your mass to calculate how many moles of copper attached to your copper strip. The molar mass of Cu is 63.54 g/mol. 2. Using your answer from step 3b, calculate how many charge carriers are in the copper ion film. 3. Knowing the total charge that was transferred to the strip (see #3a above) and how many charge carriers in the copper film, calculate the amount of charge for one of those charge carriers. This is the fundamental unit of charge. The accepted value is 1.6 X 10-19 C. Fundamental Unit of Charge. e = Percent error =Answer the following questions with your group. 1. Metal spheres 1 and 2 are touching. Both are initially neutral. a. The charged rod is brought near. 2 b. The spheres are separated. c. The charged rod is then removed. Afterward, what is the charge on each sphere? Positive, negative, or neutral? 2. Identical metal spheres are shown below. Spheres Q and R touch and then are separated. Then, P and R touch and then are separated. What is the charge on each sphere? 2 3 c= and P Q R + 4 nC -2 nC -1 nC - - 0.125 10 =125to = 0.5625 3. A 16 uC point charge is located at the origin. Another charge of 12 uC is located 1.2 meters to the right of the origin. Where would a negative point charge be placed in between these charges as to remain at equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


