Question: need help solving asap please Problem 2. (6 points) non de Calculate the entropy change for water going from state A of 1 bar and
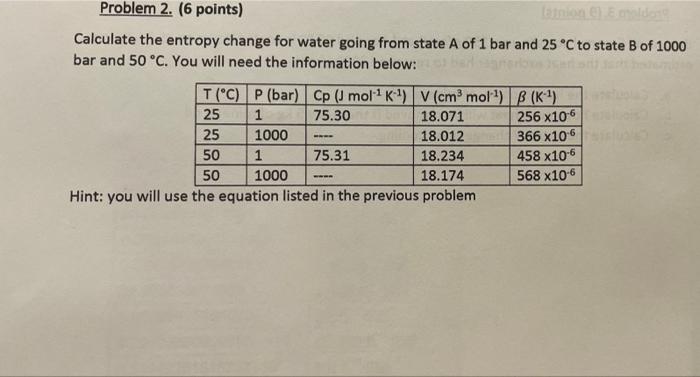
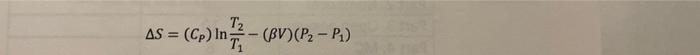
Problem 2. (6 points) non de Calculate the entropy change for water going from state A of 1 bar and 25C to state B of 1000 bar and 50 C. You will need the information below: - T("C) P (bar) Cp (mol K) V(cmmol) (K) 25 1 75.30 18.071 256 x106 25 1000 18.012 366 x106 50 1 75.31 18.234 458 x10-6 50 1000 18.174 568 x106 Hint: you will use the equation listed in the previous problem T2 AS = (Cp) in (C) in In = T (BV) (P2-P)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


