Question: need help with A and B. Relating Different Forms of the Equilibrium Constant For the reaction 2CH4(g)=C2H2(g)+3H2(g) For chemical reactions invoving lieal guses, the equlbbrum
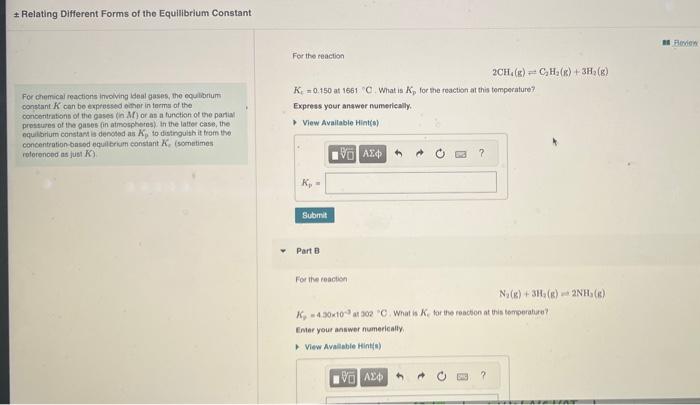
Relating Different Forms of the Equilibrium Constant For the reaction 2CH4(g)=C2H2(g)+3H2(g) For chemical reactions invoving lieal guses, the equlbbrum KF=0.150 at 1661C. What is Kp for the reaction at this temperature? constant K c can be expressed emor in torms of the concentrations of the gases (n Mf ) or at a function of the partial proseures of the gases in atmosphores) In the latter case, the nquilbrlum conctast is denosed ais Kp to distinguith it tram tho concentration based oqual bruim constant h, (sometimes relorenced as just K ). Part B For the reacien N2(g)+JH3(g)=2NH3(g) K7=4.40103 at 202C. What is Ke tor the reaction at teis tomperahara? Enter your answer numerically
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


