Question: Need help with letter D. please show step by step. 2. Consider the following reaction: 2Na(s)+O2(g)Na2O2(s)H=515kJ a. Is the reaction endothermic or exothermic? 60 thermic
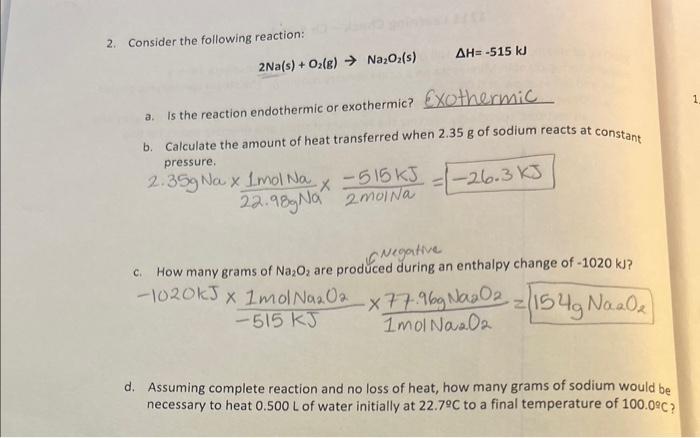
2. Consider the following reaction: 2Na(s)+O2(g)Na2O2(s)H=515kJ a. Is the reaction endothermic or exothermic? 60 thermic b. Calculate the amount of heat transferred when 2.35g of sodium reacts at constant pressure. 2.35gNa22.98Na1molNa2molNa515KJ=26.3KJ 1020kJ515KJ1molNa2N21molNa2O277.96Na2O2=154NaO2 d. Assuming complete reaction and no loss of heat, how many grams of sodium would be necessary to heat 0.500L of water initially at 22.7C to a final temperature of 100C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


