Question: In which acid-base reaction would the reactants be favored? A) HCO3(aq)+S2(aq)CO32(aq)+HS(aq) B) HCO3(aq) +HCOOH(aq)H2CO3(aq)+HCOO(aq) C) HCO3-aq +HS(aq)CO32(aq)+H2S(aq) D) HCO3(aq)+HNO2(aq)H2CO3(aq)+NO2(aq) HX is a weak acid. In
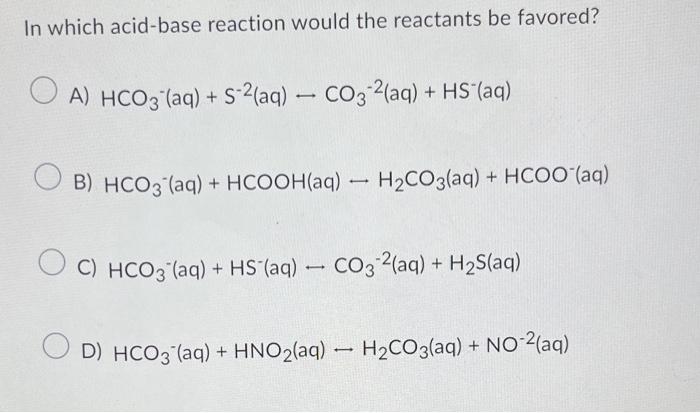

In which acid-base reaction would the reactants be favored? A) HCO3(aq)+S2(aq)CO32(aq)+HS(aq) B) HCO3(aq) +HCOOH(aq)H2CO3(aq)+HCOO(aq) C) HCO3-aq +HS(aq)CO32(aq)+H2S(aq) D) HCO3(aq)+HNO2(aq)H2CO3(aq)+NO2(aq) HX is a weak acid. In a 0.10mol/LHX(aq) solution, the species present in highest concentration is: A) OH(aq) B) HX(aq) C) H3O+(aq) D) X(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


