Question: need help with part b and C 6. A fuel mixture used in the early days of rocketry is composed of two liquids, hydrazine (N2H4)
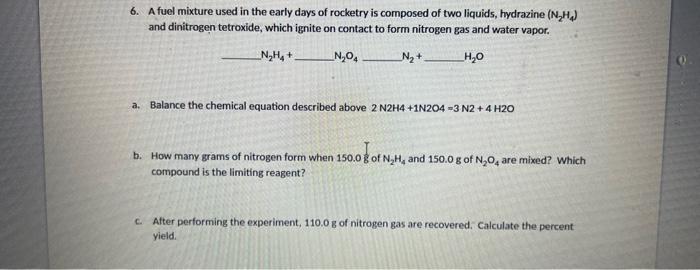
6. A fuel mixture used in the early days of rocketry is composed of two liquids, hydrazine (N2H4) and dinitrogen tetroxide, which ignite on contact to form nitrogen gas and water vapor. N2H4+N2O4N2+H2O a. Balance the chemical equation described above 2N2H4+1N2O4=3N2+4H2O b. How many grams of nitrogen form when 150.0g of N2H4 and 150.0g of N2O4 are mixed? Which compound is the limiting reagent? c. After performing the experiment, 110.0g of nitrogen gas are recovered. Calculate the percent yield
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


