Question: need help with the attached: A chemist needs to create a 25% HCl solution. (HCl is hydrochloric acid. A 25% HCl solution contains 25% HO
need help with the attached:
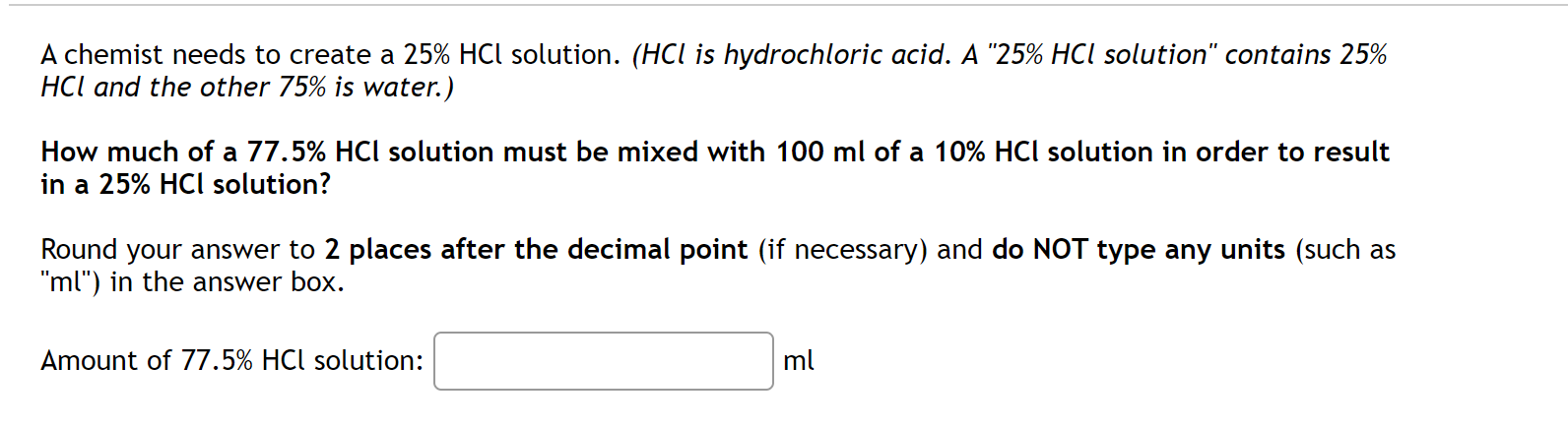
A chemist needs to create a 25% HCl solution. (HCl is hydrochloric acid. A "25% HCl solution" contains 25% HO and the other 75% is water.) How much of a 77.5% HCl solution must be mixed with 100 ml of a 10% HCl solution in order to result in a 25% HCl solution? Round your answer to 2 places after the decimal point (if necessary) and do NOT type any units (such as "ml") in the answer box. Amount of 77.5% HCl solution: :] ml
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


