Question: need help with the ISE fluoride lab, will rate. thank you! A. Determination of Calibration Slope (S) 1. Using EXCEL, plot the mV reading for

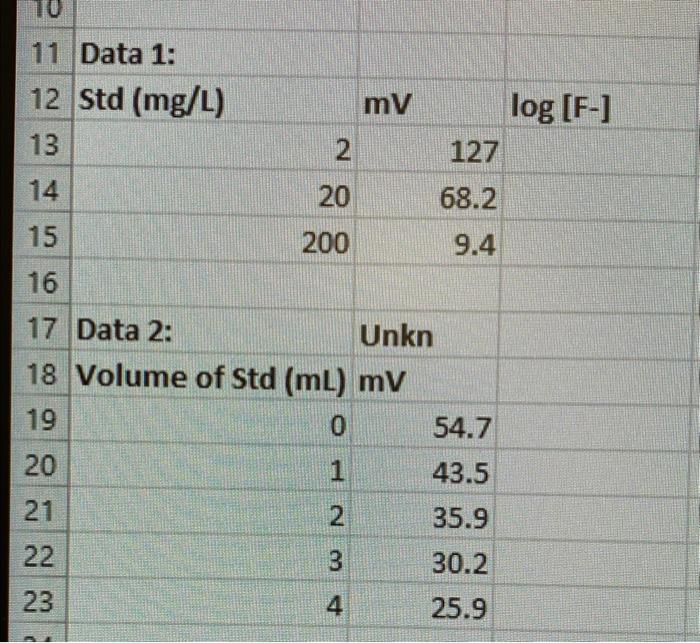
A. Determination of Calibration Slope (S) 1. Using EXCEL, plot the mV reading for the diluted fluoride standards versus the log of the actual fluoride ion concentration. 2. Fit the data points with a linear least-squares line and from the equation for the line obtain the slope (S). B. Determination of Unknown Concentration by Standard Addition 1. Using the slope determined in III.A, plot 10Es(V0+Vud) versus CudVud. Remember to include the initial reading with no added standard. 2. Fit the data points with a linear least-squares line and obtain the equation for the line. 3. Use the equation for the line to determine the x-intercept and from this calculate the fluoride ion concentration in the unknown solution. Report the fluoride ion concentration (g/mL) in the unknown solution together with the error ( SD) (calculate the standard deviation using appropriate propagation or error from a standard addition calibration curve (see textbook). 11 Data 1: A. Determination of Calibration Slope (S) 1. Using EXCEL, plot the mV reading for the diluted fluoride standards versus the log of the actual fluoride ion concentration. 2. Fit the data points with a linear least-squares line and from the equation for the line obtain the slope (S). B. Determination of Unknown Concentration by Standard Addition 1. Using the slope determined in III.A, plot 10Es(V0+Vud) versus CudVud. Remember to include the initial reading with no added standard. 2. Fit the data points with a linear least-squares line and obtain the equation for the line. 3. Use the equation for the line to determine the x-intercept and from this calculate the fluoride ion concentration in the unknown solution. Report the fluoride ion concentration (g/mL) in the unknown solution together with the error ( SD) (calculate the standard deviation using appropriate propagation or error from a standard addition calibration curve (see textbook). 11 Data 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


