Question: Need help with these 2 problems You wish to prepare 246 grams of 29.2%Cr(CH3COO)3 solution. You will need grams of chromium(III) acetate and mL of

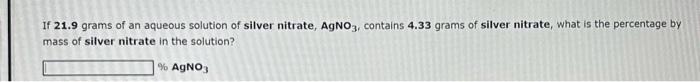
You wish to prepare 246 grams of 29.2%Cr(CH3COO)3 solution. You will need grams of chromium(III) acetate and mL of water. Assume that the density of water is 1.00g/mL. If 21.9 grams of an aqueous solution of silver nitrate, AgNO3, contains 4.33 grams of silver nitrate, what is the percentage by mass of silver nitrate in the solution? %AgNO3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


