Question: Need help with these 3 problems pls help In the laboratory you dissolve 18.8g of silver nitrate in a volumetric flask and add water to
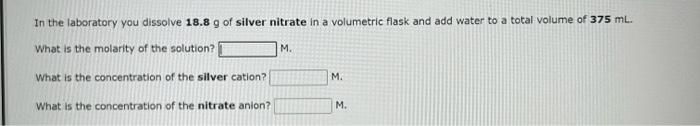
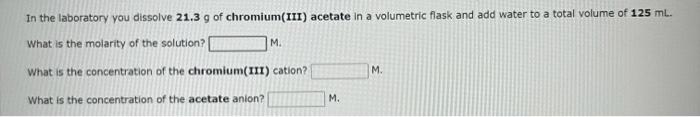

In the laboratory you dissolve 18.8g of silver nitrate in a volumetric flask and add water to a total volume of 375mL. What is the molarity of the solution? M. What is the concentration of the silver cation? M. What is the concentration of the nitrate anion? M. In the laboratory you dissolve 21.3g of chromium(III) acetate in a volumetric flask and add water to a total volume of 125mL. What is the molarity of the solution? M. What is the concentration of the chromium(III) cation? M. What is the concentration of the acetate anion? M. In the laboratory you dilute 4.36mL of a concentrated 6.00M hydrochloric acid solution to a total volume of 175mL. What is the concentration of the dilute solution? M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


