Question: Need help with these 3 problems pls helps The pH of an aqueous solution at 25C was found to be 8.40. The pOH of this
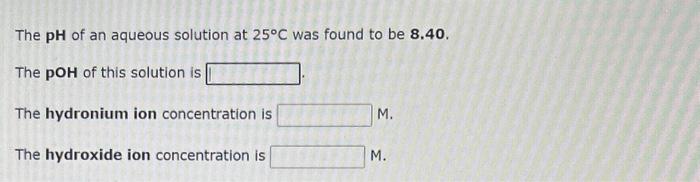


The pH of an aqueous solution at 25C was found to be 8.40. The pOH of this solution is The hydronium ion concentration is M. The hydroxide ion concentration is M. What is the pH of an aqueous solution of 2.73102M perchloric acid? What is the hydronium ion concentration in an aqueous hydroiodic acid solution with a pH of 1.050 ? [H3O+]=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


