Question: need help with these. will rate thanks 18. Predict the product of the reaction shown below. 19. Predict the product of the reaction shown below.
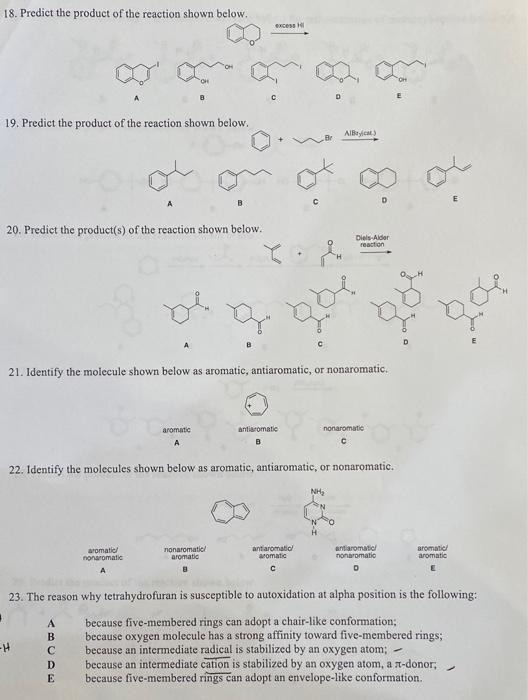
18. Predict the product of the reaction shown below. 19. Predict the product of the reaction shown below. 20. Prediet the product(s) of the reaction shown below. 21. Identify the molecule shown below as aromatic, antiaromatic, or nonaromatic. 22. Identify the molecules shown below as aromatic, antiaromatic, or nonaromatic. 23. The reason why tetrahydrofuran is susceptible to autoxidation at alpha position is the following: A because five-membered rings can adopt a chair-like conformation; B because oxygen molecule has a strong affinity toward five-membered rings; because an intermediate radical is stabilized by an oxygen atom; because five-membered rings can adopt an envelope-like conformation. 18. Predict the product of the reaction shown below. 19. Predict the product of the reaction shown below. 20. Prediet the product(s) of the reaction shown below. 21. Identify the molecule shown below as aromatic, antiaromatic, or nonaromatic. 22. Identify the molecules shown below as aromatic, antiaromatic, or nonaromatic. 23. The reason why tetrahydrofuran is susceptible to autoxidation at alpha position is the following: A because five-membered rings can adopt a chair-like conformation; B because oxygen molecule has a strong affinity toward five-membered rings; because an intermediate radical is stabilized by an oxygen atom; because five-membered rings can adopt an envelope-like conformation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


