Question: need help with these. will rate thanks 42. When D- (+)-galactose undergoes a spontancous cyclization, which oxygen atom will become a part of the six-membered
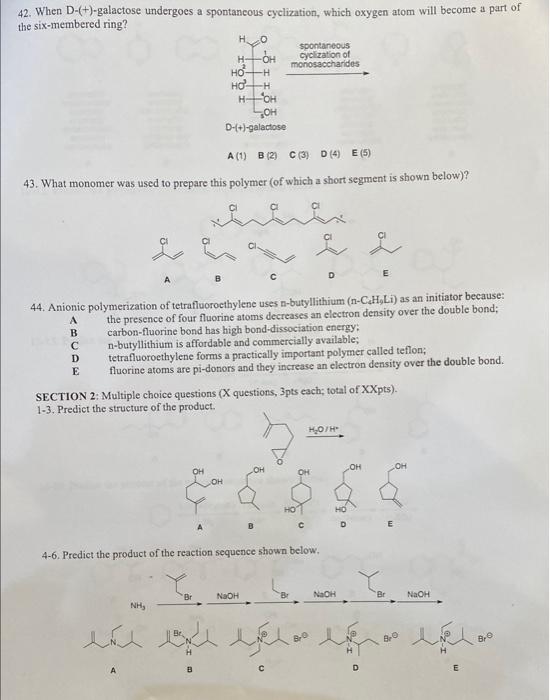
42. When D- (+)-galactose undergoes a spontancous cyclization, which oxygen atom will become a part of the six-membered ring? 43. What monomer was used to prepare this polymer (of which a short segment is shown below)? 44. Anionic polymerization of tetrafluorocthylene uses n-butyllithium (nC4H9Li) as an initiator because: A the presence of four fluorine atoms decreases an electron density over the double bond; B carbon-fluorine bond has high bond-dissociation energy: C n-butyllithium is affordable and commercially available; D tetrafluoroethylene forms a practically important polymer called teflon; E fluorine atoms are pi-donors and they increase an electron density over the double bond. SECTION 2: Multiple choice questions (X questions, 3pts each; total of XXpts). 1-3. Prediet the structure of the product. 4-6. Predict the product of the reaction sequence shown below
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


