Question: Need Help with this please thanksasap For the same reaction A - products as in the previous item, the experiment was repeated at 110C. The
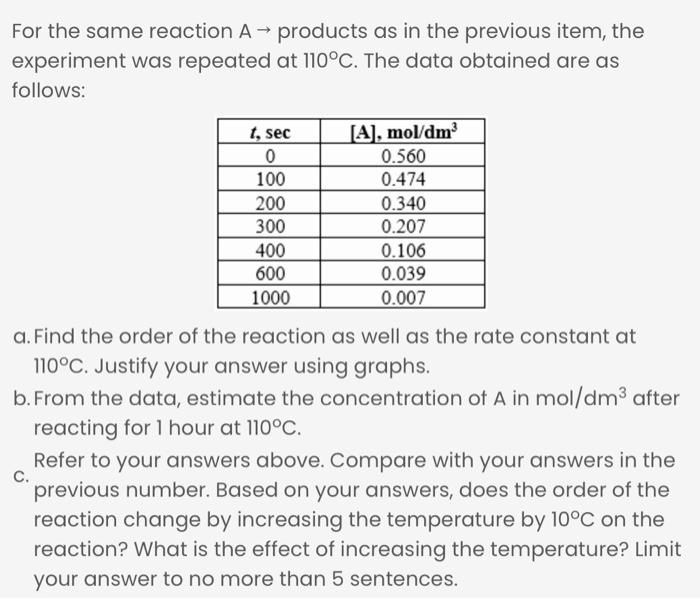
For the same reaction A - products as in the previous item, the experiment was repeated at 110C. The data obtained are as follows: t, sec 0 100 200 300 400 600 1000 (A), mol/dm 0.560 0.474 0.340 0.207 0.106 0.039 0.007 a. Find the order of the reaction as well as the rate constant at 110C. Justify your answer using graphs. b. From the data, estimate the concentration of A in mol/dm3 after reacting for 1 hour at 110C. Refer to your answers above. Compare with your answers in the C. previous number. Based on your answers, does the order of the reaction change by increasing the temperature by 10C on the reaction? What is the effect of increasing the temperature? Limit your answer to no more than 5 sentences
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


