Question: A palm oil factory uses coal as a fuel for boiler combustion. The ultimate analysis of the coal is shown in Table 1. The air
A palm oil factory uses coal as a fuel for boiler combustion. The ultimate analysis of the coal is shown in Table 1. The air supply system for the combustion is using forced draught (FD) fan. The air supply system can be regulated to achieve minimum air supply at the theoretical air required and the maximum air supply could be regulated at 75% more than theoretical air required.
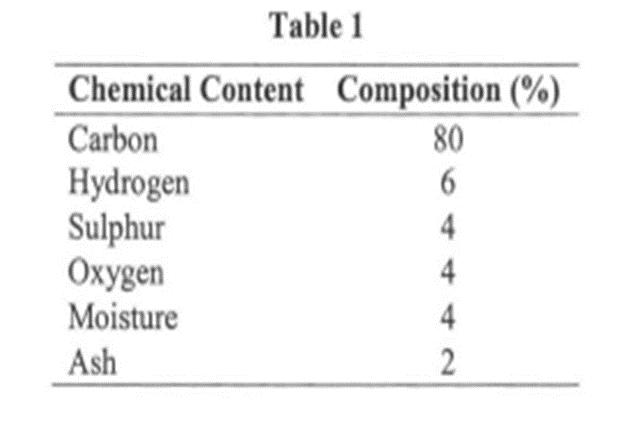
The combustion has the flue (exhaust) gas temperature is 250 oc and boiler room temperature is 33 °C. By assuming the air absolute humidity to be 0.01355 kg moisture per kg air and Cp for the gas exhaust to be 1.10 kJ/kg K, determine;
(a) The complete chemical equation for the combustion process at theoretical air supply.
(b) The percentage of air excess if the combustion process produces 02 of 15% and C02 of 20%. Assume that the C02 meter also absorbs S02.
(c) The energy carried by dry flue gas per kg fuel
Table 1 Chemical Content Composition (%) Carbon Hydrogen Sulphur Oxygen Moisture Ash 80 6 4 4 4 2
Step by Step Solution
3.56 Rating (149 Votes )
There are 3 Steps involved in it
To solve this problem lets tackle it stepbystep a Complete Chemical Equation for Combustion at Theoretical Air Supply 1 Determine the composition of c... View full answer

Get step-by-step solutions from verified subject matter experts


