Question: need some explaination as well pls. Additional Questions 1. Based on your rate law, what would happen to (i) the initial reaction rate and (ii)
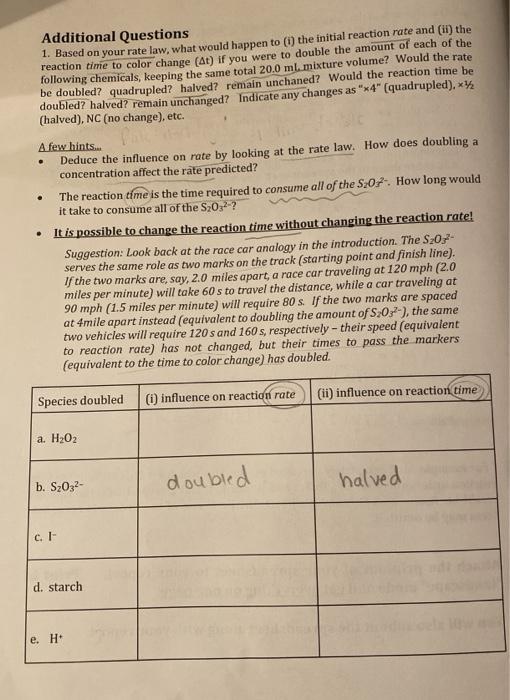
Additional Questions 1. Based on your rate law, what would happen to (i) the initial reaction rate and (ii) the reaction time to color change (At) if you were to double the amount of each of the following chemicals, keeping the same total 20.0 ml mixture volume? Would the rate be doubled? quadrupled? halved? remain unchaned? Would the reaction time be doubled? halved? remain unchanged? Indicate any changes as "x4" (quadrupled).* (halved), NC (no change), etc. . A few hints.. Deduce the influence on rate by looking at the rate law. How does doubling a concentration affect the rate predicted? The reaction time is the time required to consume all of the S:07How long would it take to consume all of the S20,2-? It is possible to change the reaction time without changing the reaction rate! Suggestion: Look back at the race car analogy in the introduction. The S20,- serves the same role as two marks on the track (starting point and finish line). If the two marks are, say, 2.0 miles apart, a race car traveling at 120 mph (2.0 miles per minute) will take 60s to travel the distance, while a car traveling at 90 mph (1.5 miles per minute) will require 80 s. If the two marks are spaced at 4mile apart instead (equivalent to doubling the amount of S:03), the same two vehicles will require 120 sand 160 s, respectively - their speed (equivalent to reaction rate) has not changed, but their times to pass the markers (equivalent to the time to color change) has doubled. Species doubled (i) influence on reaction rate (11) influence on reaction time a. H202 b. Sz032- halved doubled c. 1 d. starch e. H
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


