Question: Need to do Q3 , for more information data Q 2 also provided The following liquid-phase reactions were carried out isothermally in a CSTR at

Need to do Q3 , for more information data Q 2 also provided
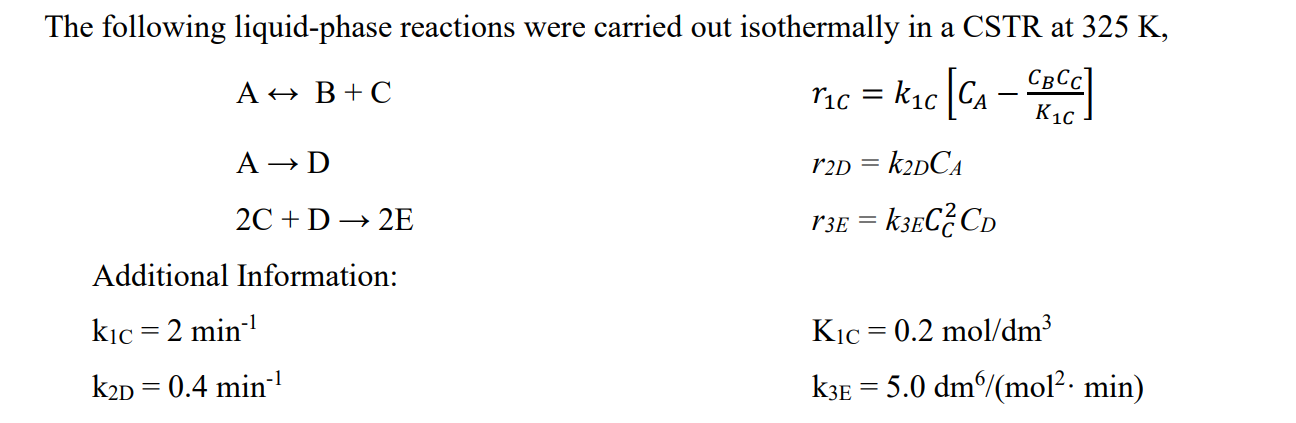
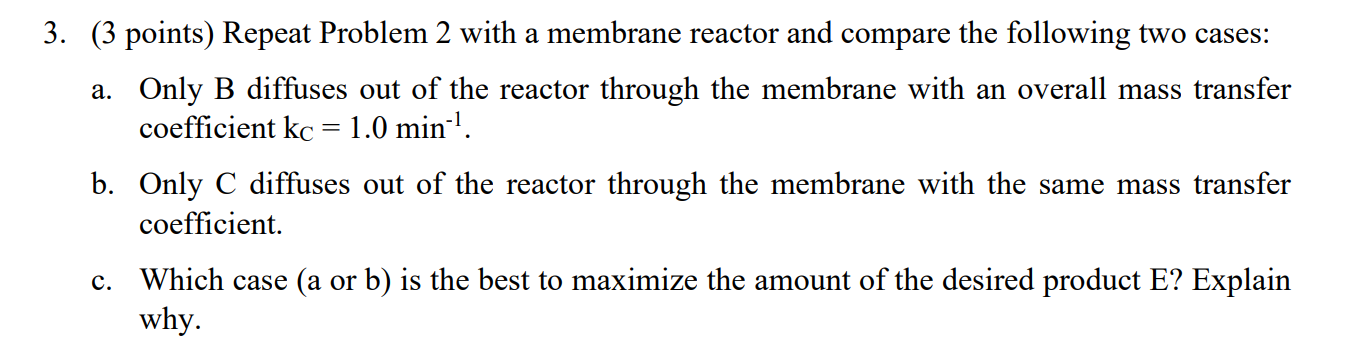
The following liquid-phase reactions were carried out isothermally in a CSTR at 325 K, CBCC A H B +C [- ric = kc [cs - Chord A D r2D = k2DCA = 2C + D + 2E r3E = k3EC2C) = Additional Information: Kic= 0.2 mol/dm3 kic = 2 min-1 k2D = 0.4 min-1 - k3E = 5.0 dm/mol2. min) 3. (3 points) Repeat Problem 2 with a membrane reactor and compare the following two cases: a. Only B diffuses out of the reactor through the membrane with an overall mass transfer coefficient kc = 1.0 min-1. b. Only C diffuses out of the reactor through the membrane with the same mass transfer coefficient. c. Which case (a or b) is the best to maximize the amount of the desired product E? Explain why. a 2. (3 points) Now assume the reactions take place in the gas phase in a PFR. Pure A enters the reactor at 30 atm and 600 K, and a flow rate of A of 15 mol/min. a. Plot the molar flow rates of each species along the volume (length) of the reactor on the same figure. b. Plot and analyze the overall: YE, Sc/d, and SD/E
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


