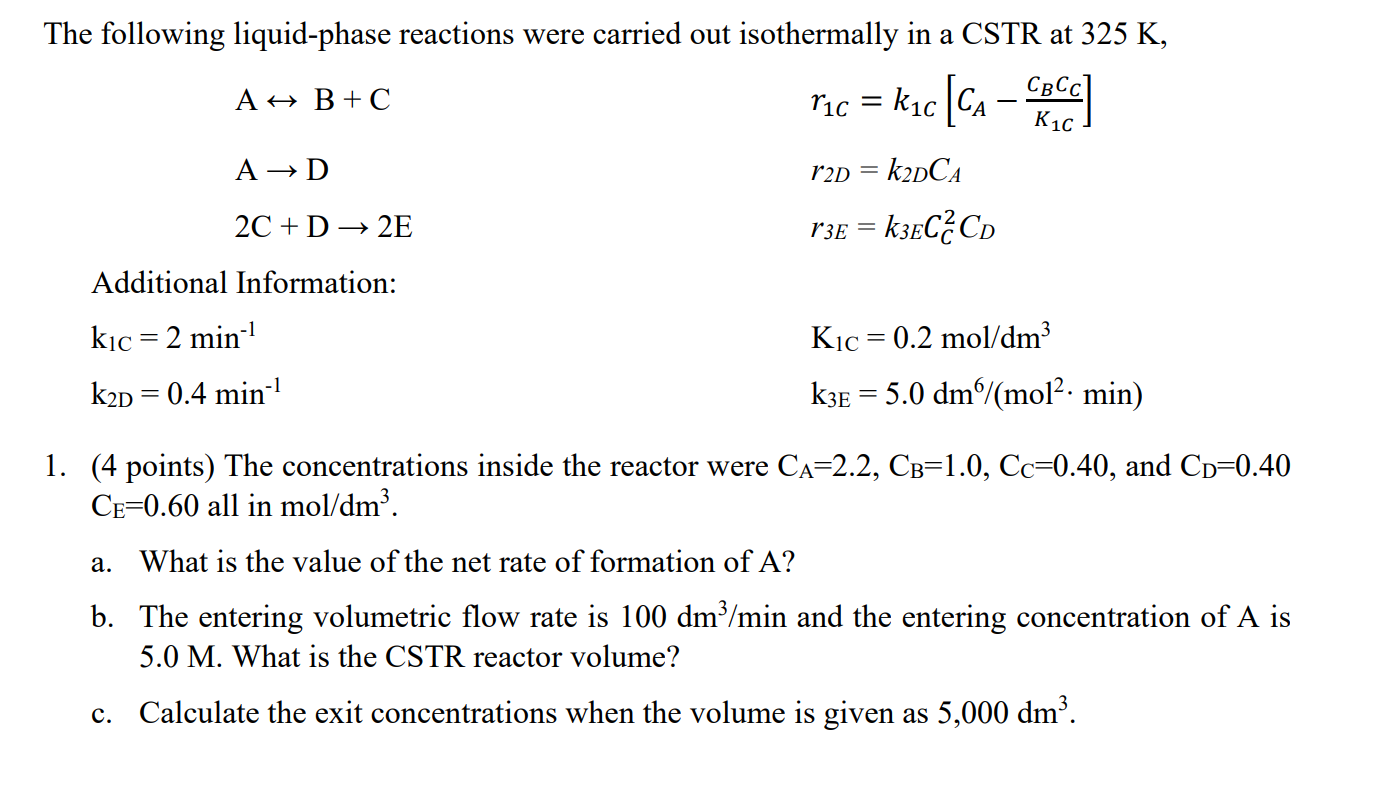
Question: Need polymath calculations and normal calculations plz guide it = K1C The following liquid-phase reactions were carried out isothermally in a CSTR at 325 K,
Need polymath calculations and normal calculations
plz guide it
= K1C The following liquid-phase reactions were carried out isothermally in a CSTR at 325 K, A H B +C c] ric = k1c [CA A D r2D = k2DCA 2C + D + 2E r3E = k3EC2CD Additional Information: kic = 2 min-1 Kic= 0.2 mol/dm3 k2D = 0.4 min-1 k3e = 5.0 dm/(mol2. min) 1. (4 points) The concentrations inside the reactor were Ca=2.2, CB=1.0, Cc=0.40, and CD=0.40 CE=0.60 all in mol/dm3. a. What is the value of the net rate of formation of A? b. The entering volumetric flow rate is 100 dm3/min and the entering concentration of A is 5.0 M. What is the CSTR reactor volume? c. Calculate the exit concentrations when the volume is given as 5,000 dm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


