Question: needed graph and curve and explanation please do it urgent needed Practical Exercise 3- BUFFERS, pH MEASUREMENT AND AMINO ACID TITRATION Results Sheet Exercise 3.1.
 needed graph and curve and explanation please do it urgent needed
needed graph and curve and explanation please do it urgent needed
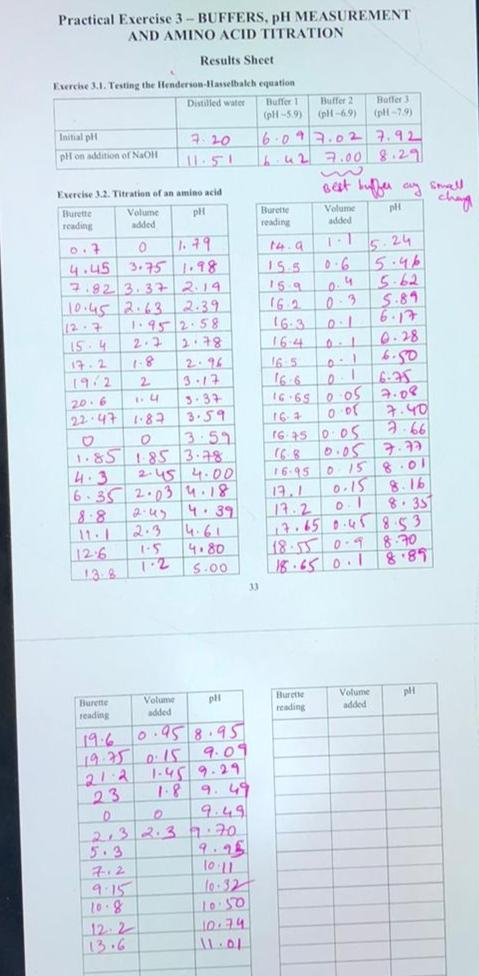
Practical Exercise 3- BUFFERS, pH MEASUREMENT AND AMINO ACID TITRATION Results Sheet Exercise 3.1. Testing the Henderson-Hasselbalch equation Distilled water Buffer 1 Buffer 2 Baffer 3 (pH-5.9) (pH-6.9) (pH-7.9) Initial pH 7:20 6.09 7.02 7.92 pH on addition of NaOH 6.42 7.00 8.29 Exercise 3.2. Titration of an amino acid Burette Volume pH Burette reading added reading 0.7 0 1.79 4.45 3.75 1.98 7.82 3.37 2.19 10.45 2.63. 2.39 12-7 1.95 2.58 15-4 2.2 2.78 17.2 1-8 2.96 19/2 2 3.17 20.6 1.4 3.37 22.47 1-87 3.59 0 3.59 1.85 1.85 3.78 4.3 2.45 4.00 6.35 2.03 4.18 2.45 4.39 11-1 2.3 4.61 12.6 1-5 4.80 1.2 pli Burette reading Volume added 19.6 0.95 8.95 19.75 0.15 9.09 21:2 1.45 9.29 23 1.8 D O 9.49 2.3 2.3 77.70 9.25 7.2 10:11 9.15 10.32 10.8 10:50 12 2 10.74 13.6 11.01 138 5.00 E 33 14. a 155 159 16.2 + Best buffer ay sowell Volume pH added 7-1 5.24 0.6 5.46 0.4 5-62 0-3 5.89 6.17 0.28 6.50 16-3 164 16 5 16.65 0.05 16-4 0:01 16:45 0.05 7.77 16.95 0 15 8.01 13.1 0.15 8.16 17.2 0.1 8.35 17.65 0.45 853 8.70 18.55 0.9 18.65 0.1 8.89 Burette Volume added pH reading 6-75 7.08 7.40 2-66 chay
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


