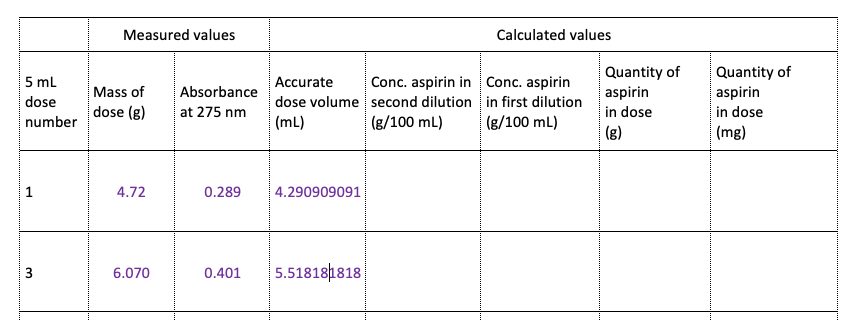
Question: Needing help with dillution factor. Information - First dilution: dilute the dose to 1 0 0 mL . Transfer the dose from the medicine measure
Needing help with dillution factor.
Information
First dilution: dilute the dose to mL Transfer the dose from the medicine
measure into a mL volumetric flask. Use pH buffer to rinse the measure and
transfer rinsings to the volumetric flask, then add more buffer to bring the volume up
to the mark.
Second dilution: Make a further in dilution using the volumetric flasks available
to you. For example, mL in mL or mL in mL or mL in mL Use
pipettors to accurately transfer the volume required.
Determine the absorbance of this solution at nm and record the result.
Absorbance value calculated from data is concentration in g mL absorbance
Therefore for concentration is gml & gml
Please help fill dilution factor of table below
tableMeasured values,Calculated valuestable
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


