Question: Nernst Ecell What is the calculated value of the cell potential at 298K for an electrochemical cell with the following reaction, when the Pb2+ concentration


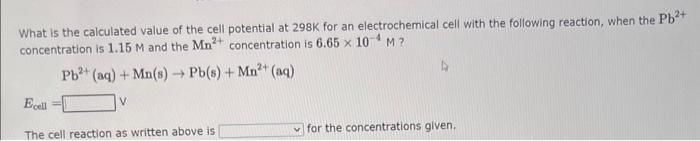
What is the calculated value of the cell potential at 298K for an electrochemical cell with the following reaction, when the Pb2+ concentration is 7.92104M and the Cr3+ concentration is 1.32M ? Ecell=V3Pb2+(aq)+2Cr(s)3Pb(s)+2Cr3+(aq) The cell reaction as written above is for the concentrations given. What is the calculated value of the cell potential at 298K for an electrochemical cell with the following reaction, when the Pb2+ concentration is 1.41M and the Al3+ concentration is 4.05104M ? Ecall=v3Pb2+(aq)+2Al(s)+3Pb(s)+2Al3+(aq) The cell reaction as written above is for the concentrations given. What is the calculated value of the cell potential at 298K for an electrochemical cell with the following reaction, when the Pb2+ concentration is 1.15M and the Mn2+ concentration is 6.65104M ? Pb2+(aq)+Mn(s)Pb(s)+Mn2+(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


