Question: i need help for the three questions When the Ag+concentration is 9.59104M, the observed cell potential at 298K for an electrochemical cell with the following
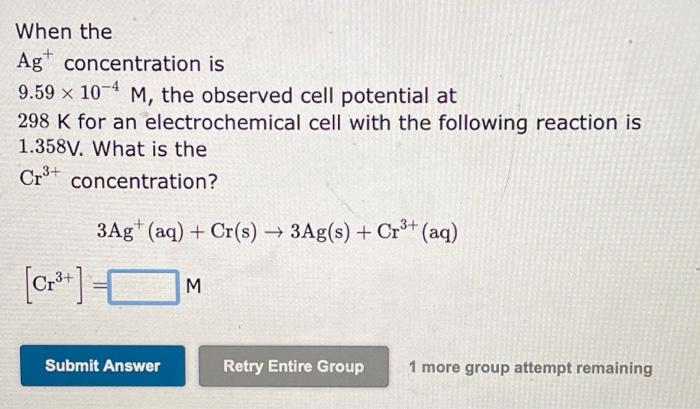
![following reaction is 1.358V. What is the Cr3+ concentration? 3Ag+(aq)+Cr(s)3Ag(s)+Cr3+(aq)[Cr3+]= 1 more](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f96cc951db4_34466f96cc8e2d71.jpg)
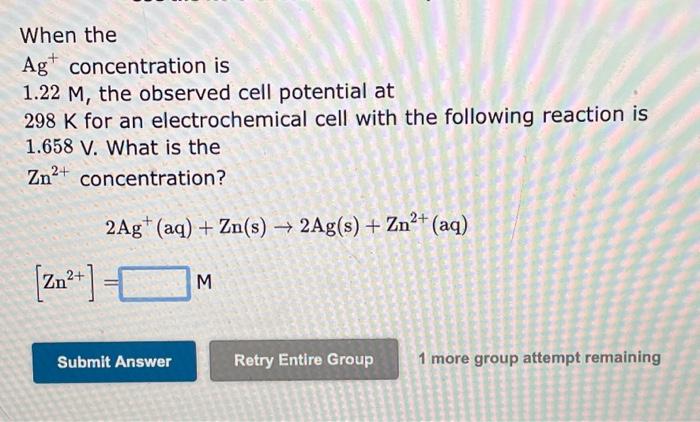
When the Ag+concentration is 9.59104M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 1.358V. What is the Cr3+ concentration? 3Ag+(aq)+Cr(s)3Ag(s)+Cr3+(aq)[Cr3+]= 1 more group attempt remaining When the Ag+concentration is 1.22M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 1.658V. What is the Zn2+ concentration? 2Ag+(aq)+Zn(s)2Ag(s)+Zn2+(aq)[Zn2+]=M 1 more group attempt remaining When the Ag+concentration is 1.31M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 3.275V. What is the Mg2+ concentration? 2Ag+(aq)+Mg(s)2Ag(s)+Mg2+(aq)[Mg2+]=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


