Question: Nicotine (C) in a water (A) solution containing 1% nicotine is to be extracted with kerosene (B) at 20C. Water and kerosene are essentially insoluble.
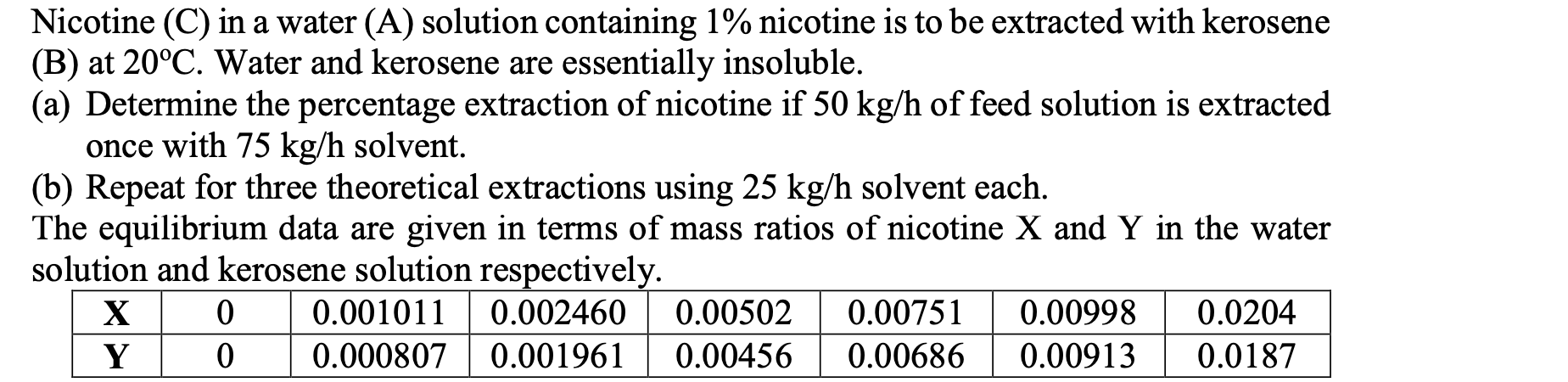
Nicotine (C) in a water (A) solution containing 1% nicotine is to be extracted with kerosene (B) at 20C. Water and kerosene are essentially insoluble. (a) Determine the percentage extraction of nicotine if 50 kg/h of feed solution is extracted once with 75 kg/h solvent. (b) Repeat for three theoretical extractions using 25 kg/h solvent each. The equilibrium data are given in terms of mass ratios of nicotine X and Y in the water solution and kerosene solution respectively. X 0 0.001011 0.002460 0.00502 0.00751 0.00998 0.0204 Y 0 0.000807 0.001961 0.00456 0.00686 0.00913 0.0187
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


