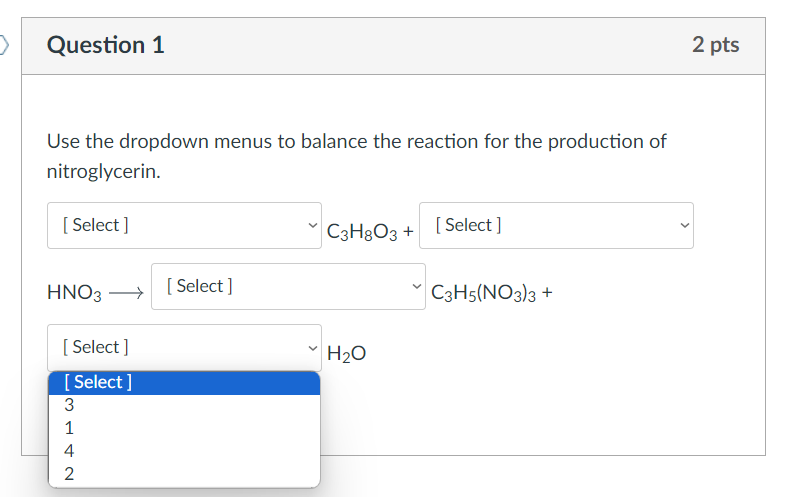
Question: Nitroglycerin ( C 3 H 5 ( NO 3 ) 3 ) is produced by adding concentrated nitric acid ( HNO 3 ) to glycerin
Nitroglycerin CHNO is produced by adding concentrated nitric acid HNO to glycerin CHO
In the past, this was performed as a semibatch operation in a large stirred pot containing ~ ton of material. The pot was initially filled with glycerin and the acid was then added slowly over a hour period. Nitroglycerin might explore if heat from the reaction is not removed. The story is told that operators used to sit on onelegged stools to make sure they didn't fall asleep while watching the pot. Now, a safer continuousflow process has been developed that reduces the size of the reactor and the reaction time.
In this simple exercise, you just need to determine the heat of reactions for nitroglycerin production and explosive decomposition.
Please use kJmol for the enthalpy of formation for liquid nitroglycerin. One version of the table in the book has the value equal to which is the value for gas phase.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


